Dysregulation of innate and adaptive serum mediators precedes systemic lupus erythematosus classification and improves prognostic accuracy of autoantibodies
- PMID: 27338520
- PMCID: PMC5079766
- DOI: 10.1016/j.jaut.2016.06.001
Dysregulation of innate and adaptive serum mediators precedes systemic lupus erythematosus classification and improves prognostic accuracy of autoantibodies
Abstract
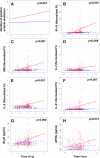
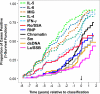
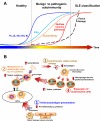
Systemic lupus erythematosus (SLE) is a complex autoimmune disease with a poorly understood preclinical stage of immune dysregulation and symptom accrual. Accumulation of antinuclear autoantibody (ANA) specificities is a hallmark of impending clinical disease. Yet, many ANA-positive individuals remain healthy, suggesting that additional immune dysregulation underlies SLE pathogenesis. Indeed, we have recently demonstrated that interferon (IFN) pathways are dysregulated in preclinical SLE. To determine if other forms of immune dysregulation contribute to preclinical SLE pathogenesis, we measured SLE-associated autoantibodies and soluble mediators in samples from 84 individuals collected prior to SLE classification (average timespan = 5.98 years), compared to unaffected, healthy control samples matched by race, gender, age (±5 years), and time of sample procurement. We found that multiple soluble mediators, including interleukin (IL)-5, IL-6, and IFN-γ, were significantly elevated in cases compared to controls more than 3.5 years pre-classification, prior to or concurrent with autoantibody positivity. Additional mediators, including innate cytokines, IFN-associated chemokines, and soluble tumor necrosis factor (TNF) superfamily mediators increased longitudinally in cases approaching SLE classification, but not in controls. In particular, levels of B lymphocyte stimulator (BLyS) and a proliferation-inducing ligand (APRIL) were comparable in cases and controls until less than 10 months pre-classification. Over the entire pre-classification period, random forest models incorporating ANA and anti-Ro/SSA positivity with levels of IL-5, IL-6, and the IFN-γ-induced chemokine, MIG, distinguished future SLE patients with 92% (±1.8%) accuracy, compared to 78% accuracy utilizing ANA positivity alone. These data suggest that immune dysregulation involving multiple pathways contributes to SLE pathogenesis. Importantly, distinct immunological profiles are predictive for individuals who will develop clinical SLE and may be useful for delineating early pathogenesis, discovering therapeutic targets, and designing prevention trials.
Keywords: Autoantibodies; Biomarkers; Cytokines; Disease progression; Forecasting; Systemic lupus erythematosus.
Copyright © 2016 Elsevier Ltd. All rights reserved.
Figures

Similar articles
-
Altered type II interferon precedes autoantibody accrual and elevated type I interferon activity prior to systemic lupus erythematosus classification.Ann Rheum Dis. 2016 Nov;75(11):2014-2021. doi: 10.1136/annrheumdis-2015-208140. Epub 2016 Jan 25. Ann Rheum Dis. 2016. PMID: 27088255 Free PMC article.
-
Autoantibody-Positive Healthy Individuals Display Unique Immune Profiles That May Regulate Autoimmunity.Arthritis Rheumatol. 2016 Oct;68(10):2492-502. doi: 10.1002/art.39706. Arthritis Rheumatol. 2016. PMID: 27059145 Free PMC article.
-
BAFF-R and TACI expression on CD3+ T cells: Interplay among BAFF, APRIL and T helper cytokines profile in systemic lupus erythematosus.Cytokine. 2019 Feb;114:115-127. doi: 10.1016/j.cyto.2018.11.008. Epub 2018 Nov 19. Cytokine. 2019. PMID: 30467093
-
Autoantibodies in SLE: prediction and the p value matrix.Lupus. 2019 Oct;28(11):1285-1293. doi: 10.1177/0961203319868531. Epub 2019 Aug 9. Lupus. 2019. PMID: 31399014 Review.
-
Role of the innate and adaptive immune responses in the pathogenesis of systemic lupus erythematosus.Inflamm Res. 2022 Jun;71(5-6):537-554. doi: 10.1007/s00011-022-01554-6. Epub 2022 Mar 17. Inflamm Res. 2022. PMID: 35298669 Review.
Cited by
-
The Role of Sirtuin-1 in Immune Response and Systemic Lupus Erythematosus.Front Immunol. 2021 Apr 26;12:632383. doi: 10.3389/fimmu.2021.632383. eCollection 2021. Front Immunol. 2021. PMID: 33981300 Free PMC article. Review.
-
scFTD-seq: freeze-thaw lysis based, portable approach toward highly distributed single-cell 3' mRNA profiling.Nucleic Acids Res. 2019 Feb 20;47(3):e16. doi: 10.1093/nar/gky1173. Nucleic Acids Res. 2019. PMID: 30462277 Free PMC article.
-
SLE: reconciling heterogeneity.Lupus Sci Med. 2019 Feb 4;6(1):e000280. doi: 10.1136/lupus-2018-000280. eCollection 2019. Lupus Sci Med. 2019. PMID: 31080630 Free PMC article. No abstract available.
-
Immunological Involvement of MicroRNAs in the Key Events of Systemic Lupus Erythematosus.Front Immunol. 2021 Aug 2;12:699684. doi: 10.3389/fimmu.2021.699684. eCollection 2021. Front Immunol. 2021. PMID: 34408748 Free PMC article. Review.
-
Developing and Refining New Candidate Criteria for Systemic Lupus Erythematosus Classification: An International Collaboration.Arthritis Care Res (Hoboken). 2018 Apr;70(4):571-581. doi: 10.1002/acr.23317. Epub 2018 Feb 22. Arthritis Care Res (Hoboken). 2018. PMID: 28692774 Free PMC article. Review.
References
-
- Tsokos GC. Systemic lupus erythematosus. N Engl J Med. 2011;365:2110–21. - PubMed
-
- Arbuckle MR, McClain MT, Rubertone MV, Scofield RH, Dennis GJ, James JA, et al. Development of autoantibodies before the clinical onset of systemic lupus erythematosus. N Engl J Med. 2003;349:1526–33. - PubMed
-
- McClain MT, Heinlen LD, Dennis GJ, Roebuck J, Harley JB, James JA. Early events in lupus humoral autoimmunity suggest initiation through molecular mimicry. Nat Med. 2005;11:85–9. - PubMed
-
- James JA, Kim-Howard XR, Bruner BF, Jonsson MK, McClain MT, Arbuckle MR, et al. Hydroxychloroquine sulfate treatment is associated with later onset of systemic lupus erythematosus. Lupus. 2007;16:401–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- U54 GM104938/GM/NIGMS NIH HHS/United States
- U19 AI082714/AI/NIAID NIH HHS/United States
- P30 GM103510/GM/NIGMS NIH HHS/United States
- P30 AR053483/AR/NIAMS NIH HHS/United States
- R21 AI103980/AI/NIAID NIH HHS/United States
- U01 HG006828/HG/NHGRI NIH HHS/United States
- R37 AI024717/AI/NIAID NIH HHS/United States
- U01 AI101934/AI/NIAID NIH HHS/United States
- R01 AI024717/AI/NIAID NIH HHS/United States
- U01 HG008666/HG/NHGRI NIH HHS/United States
- P01 AR048929/AR/NIAMS NIH HHS/United States
- UL1 TR000077/TR/NCATS NIH HHS/United States
- UL1 TR001417/TR/NCATS NIH HHS/United States
- P01 AI083194/AI/NIAID NIH HHS/United States
- T32 AI007633/AI/NIAID NIH HHS/United States
- P01 AR049084/AR/NIAMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
