A non-canonical role for desmoglein-2 in endothelial cells: implications for neoangiogenesis
- PMID: 27338829
- PMCID: PMC5026727
- DOI: 10.1007/s10456-016-9520-y
A non-canonical role for desmoglein-2 in endothelial cells: implications for neoangiogenesis
Abstract
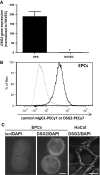
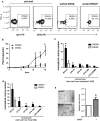
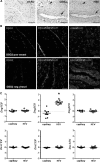
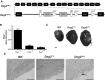
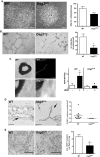
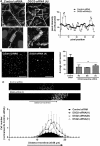
Desmogleins (DSG) are a family of cadherin adhesion proteins that were first identified in desmosomes and provide cardiomyocytes and epithelial cells with the junctional stability to tolerate mechanical stress. However, one member of this family, DSG2, is emerging as a protein with additional biological functions on a broader range of cells. Here we reveal that DSG2 is expressed by non-desmosome-forming human endothelial progenitor cells as well as their mature counterparts [endothelial cells (ECs)] in human tissue from healthy individuals and cancer patients. Analysis of normal blood and bone marrow showed that DSG2 is also expressed by CD34(+)CD45(dim) hematopoietic progenitor cells. An inability to detect other desmosomal components, i.e., DSG1, DSG3 and desmocollin (DSC)2/3, on these cells supports a solitary role for DSG2 outside of desmosomes. Functionally, we show that CD34(+)CD45(dim)DSG2(+) progenitor cells are multi-potent and pro-angiogenic in vitro. Using a 'knockout-first' approach, we generated a Dsg2 loss-of-function strain of mice (Dsg2 (lo/lo)) and observed that, in response to reduced levels of Dsg2: (i) CD31(+) ECs in the pancreas are hypertrophic and exhibit altered morphology, (ii) bone marrow-derived endothelial colony formation is impaired, (iii) ex vivo vascular sprouting from aortic rings is reduced, and (iv) vessel formation in vitro and in vivo is attenuated. Finally, knockdown of DSG2 in a human bone marrow EC line reveals a reduction in an in vitro angiogenesis assay as well as relocalisation of actin and VE-cadherin away from the cell junctions, reduced cell-cell adhesion and increased invasive properties by these cells. In summary, we have identified DSG2 expression in distinct progenitor cell subpopulations and show that, independent from its classical function as a component of desmosomes, this cadherin also plays a critical role in the vasculature.
Keywords: DESMOGLEIN-2; Endothelial progenitor cells; Endothelium; Hematopoietic stem and progenitor cells; Mouse models of neoangiogenesis.
Conflict of interest statement
The authors indicate no potential conflicts of interest.
Figures




References
-
- Pilichou K, Remme CA, Basso C, Campian ME, Rizzo S, Barnett P, Scicluna BP, Bauce B, van den Hoff MJ, de Bakker JM, Tan HL, Valente M, Nava A, Wilde AA, Moorman AF, Thiene G, Bezzina CR. Myocyte necrosis underlies progressive myocardial dystrophy in mouse dsg2-related arrhythmogenic right ventricular cardiomyopathy. J Exp Med. 2009;206(8):1787–1802. doi: 10.1084/jem.20090641. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

