Proteomic differences in brain vessels of Alzheimer's disease mice: Normalization by PPARγ agonist pioglitazone
- PMID: 27339263
- PMCID: PMC5363486
- DOI: 10.1177/0271678X16655172
Proteomic differences in brain vessels of Alzheimer's disease mice: Normalization by PPARγ agonist pioglitazone
Abstract
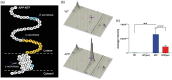
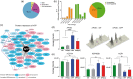
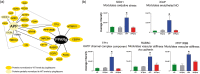
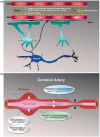
Cerebrovascular insufficiency appears years prior to clinical symptoms in Alzheimer's disease. The soluble, highly toxic amyloid-β species, generated from the amyloidogenic processing of amyloid precursor protein, are known instigators of the chronic cerebrovascular insufficiency observed in both Alzheimer's disease patients and transgenic mouse models. We have previously demonstrated that pioglitazone potently reverses cerebrovascular impairments in a mouse model of Alzheimer's disease overexpressing amyloid-β. In this study, we sought to characterize the effects of amyloid-β overproduction on the cerebrovascular proteome; determine how pioglitazone treatment affected the altered proteome; and analyze the relationship between normalized protein levels and recovery of cerebrovascular function. Three-month-old wildtype and amyloid precursor protein mice were treated with pioglitazone- (20 mg/kg/day, 14 weeks) or control-diet. Cerebral arteries were surgically isolated, and extracted proteins analyzed by gel-free and gel-based mass spectrometry. 193 cerebrovascular proteins were abnormally expressed in amyloid precursor protein mice. Pioglitazone treatment rescued a third of these proteins, mainly those associated with oxidative stress, promotion of cerebrovascular vasocontractile tone, and vascular compliance. Our results demonstrate that amyloid-β overproduction perturbs the cerebrovascular proteome. Recovery of cerebrovascular function with pioglitazone is associated with normalized levels of key proteins in brain vessel function, suggesting that pioglitazone-responsive cerebrovascular proteins could be early biomarkers of Alzheimer's disease.
Keywords: Amyloid peptide; cerebral artery; oxidative stress; proliferator-activated receptor gamma; vascular biomarkers.
Figures
References
-
- Ruitenberg A, den Heijer T, Bakker SL, et al. Cerebral hypoperfusion and clinical onset of dementia: the Rotterdam Study. Ann Neurol 2005; 57: 789–794. - PubMed
-
- Kalaria RN. Cerebral vessels in ageing and Alzheimer's disease. Pharmacol Ther 1996; 72: 193–214. - PubMed
-
- Farkas E, Luiten PG. Cerebral microvascular pathology in aging and Alzheimer's disease. Prog Neurobiol 2001; 64: 575–611. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

