The Phosphorylation and Distribution of Cortactin Downstream of Integrin α9β1 Affects Cancer Cell Behaviour
- PMID: 27339664
- PMCID: PMC4919783
- DOI: 10.1038/srep28529
The Phosphorylation and Distribution of Cortactin Downstream of Integrin α9β1 Affects Cancer Cell Behaviour
Abstract
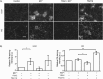
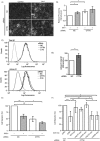
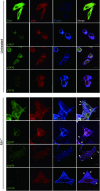
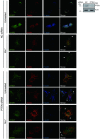
Integrins, a family of heterodimeric adhesion receptors are implicated in cell migration, development and cancer progression. They can adopt conformations that reflect their activation states and thereby impact adhesion strength and migration. Integrins in an intermediate activation state may be optimal for migration and we have shown previously that fully activated integrin α9β1 corresponds with less migratory behaviour in melanoma cells. Here, we aimed to identify components associated with the activation status of α9β1. Using cancer cell lines with naturally occuring high levels of this integrin, activation by α9β1-specific ligands led to upregulation of fibronectin matrix assembly and tyrosine phosphorylation of cortactin on tyrosine 470 (Y470). Specifically, cortactin phosphorylated on Y470, but not Y421, redistributed together with α9β1 to focal adhesions where active β1 integrin also localises, upon integrin activation. This was commensurate with reduced migration. The localisation and phosphorylation of cortactin Y470 was regulated by Yes kinase and PTEN phosphatase. Cortactin levels influenced fibronectin matrix assembly and active β1 integrin on the cell surface, being inversely correlated with migratory behaviour. This study underlines the complex interplay between cortactin and α9β1 integrin that regulates cell-extracellular matrix interactions.
Figures


Similar articles
-
Alpha9beta1 integrin in melanoma cells can signal different adhesion states for migration and anchorage.Exp Cell Res. 2009 Nov 15;315(19):3312-24. doi: 10.1016/j.yexcr.2009.09.022. Epub 2009 Sep 29. Exp Cell Res. 2009. PMID: 19796635
-
Fer-mediated cortactin phosphorylation is associated with efficient fibroblast migration and is dependent on reactive oxygen species generation during integrin-mediated cell adhesion.Mol Cell Biol. 2007 Sep;27(17):6140-52. doi: 10.1128/MCB.01744-06. Epub 2007 Jul 2. Mol Cell Biol. 2007. PMID: 17606629 Free PMC article.
-
Membrane-type 1 matrix metalloproteinase modulates focal adhesion stability and cell migration.Exp Cell Res. 2006 May 1;312(8):1381-9. doi: 10.1016/j.yexcr.2006.01.008. Epub 2006 Feb 13. Exp Cell Res. 2006. PMID: 16473349
-
Integrins in cell adhesion and signaling.Hum Cell. 1996 Sep;9(3):181-6. Hum Cell. 1996. PMID: 9183647 Review.
-
Dimensions and dynamics in integrin function.Braz J Med Biol Res. 2003 Aug;36(8):959-66. doi: 10.1590/s0100-879x2003000800001. Epub 2003 Jul 23. Braz J Med Biol Res. 2003. PMID: 12886449 Review.
Cited by
-
Cortactin in Lung Cell Function and Disease.Int J Mol Sci. 2022 Apr 21;23(9):4606. doi: 10.3390/ijms23094606. Int J Mol Sci. 2022. PMID: 35562995 Free PMC article. Review.
-
A single tyrosine phosphorylation site in cortactin is important for filopodia formation in neuronal growth cones.Mol Biol Cell. 2019 Jul 15;30(15):1817-1833. doi: 10.1091/mbc.E18-04-0202. Epub 2019 May 22. Mol Biol Cell. 2019. PMID: 31116646 Free PMC article.
-
Requirement of Site-Specific Tyrosine Phosphorylation of Cortactin in Retinal Neovascularization and Vascular Leakage.Arterioscler Thromb Vasc Biol. 2024 Feb;44(2):366-390. doi: 10.1161/ATVBAHA.123.320279. Epub 2023 Dec 21. Arterioscler Thromb Vasc Biol. 2024. PMID: 38126170 Free PMC article.
-
The characteristics and the multiple functions of integrin β1 in human cancers.J Transl Med. 2023 Nov 6;21(1):787. doi: 10.1186/s12967-023-04696-1. J Transl Med. 2023. PMID: 37932738 Free PMC article. Review.
References
-
- Hynes R. O. Integrins: bidirectional, allosteric signaling machines. Cell 110, 673–687 (2002). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

