SAS6-like protein in Plasmodium indicates that conoid-associated apical complex proteins persist in invasive stages within the mosquito vector
- PMID: 27339728
- PMCID: PMC4919640
- DOI: 10.1038/srep28604
SAS6-like protein in Plasmodium indicates that conoid-associated apical complex proteins persist in invasive stages within the mosquito vector
Abstract
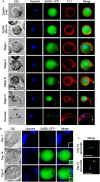
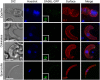
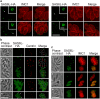
The SAS6-like (SAS6L) protein, a truncated paralogue of the ubiquitous basal body/centriole protein SAS6, has been characterised recently as a flagellum protein in trypanosomatids, but associated with the conoid in apicomplexan Toxoplasma. The conoid has been suggested to derive from flagella parts, but is thought to have been lost from some apicomplexans including the malaria-causing genus Plasmodium. Presence of SAS6L in Plasmodium, therefore, suggested a possible role in flagella assembly in male gametes, the only flagellated stage. Here, we have studied the expression and role of SAS6L throughout the Plasmodium life cycle using the rodent malaria model P. berghei. Contrary to a hypothesised role in flagella, SAS6L was absent during gamete flagellum formation. Instead, SAS6L was restricted to the apical complex in ookinetes and sporozoites, the extracellular invasive stages that develop within the mosquito vector. In these stages SAS6L forms an apical ring, as we show is also the case in Toxoplasma tachyzoites. The SAS6L ring was not apparent in blood-stage invasive merozoites, indicating that the apical complex is differentiated between the different invasive forms. Overall this study indicates that a conoid-associated apical complex protein and ring structure is persistent in Plasmodium in a stage-specific manner.
Figures


References
-
- Leidel S., Delattre M., Cerutti L., Baumer K. & Gonczy P. SAS-6 defines a protein family required for centrosome duplication in C. elegans and in human cells. Nat. Cell Biol. 7, 115–125 (2005). - PubMed
-
- Nakazawa Y., Hiraki M., Kamiya R. & Hirono M. SAS-6 is a cartwheel protein that establishes the 9-fold symmetry of the centriole. Curr. Biol. 17, 2169–2174 (2007). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

