Gut microbiota drive the development of neuroinflammatory response in cirrhosis in mice
- PMID: 27339732
- PMCID: PMC5033692
- DOI: 10.1002/hep.28696
Gut microbiota drive the development of neuroinflammatory response in cirrhosis in mice
Abstract
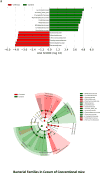
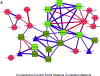
The mechanisms behind the development of hepatic encephalopathy (HE) are unclear, although hyperammonemia and systemic inflammation through gut dysbiosis have been proposed. The aim of this work was to define the individual contribution of hyperammonemia and systemic inflammation on neuroinflammation in cirrhosis using germ-free (GF) and conventional mice. GF and conventional C57BL/6 mice were made cirrhotic using CCl4 gavage. These were compared to their noncirrhotic counterparts. Intestinal microbiota, systemic and neuroinflammation (including microglial and glial activation), serum ammonia, intestinal glutaminase activity, and cecal glutamine content were compared between groups. GF cirrhotic mice developed similar cirrhotic changes to conventional mice after 4 extra weeks (16 vs. 12 weeks) of CCl4 gavage. GF cirrhotic mice exhibited higher ammonia, compared to GF controls, but this was not associated with systemic or neuroinflammation. Ammonia was generated through increased small intestinal glutaminase activity with concomitantly reduced intestinal glutamine levels. However, conventional cirrhotic mice had intestinal dysbiosis as well as systemic inflammation, associated with increased serum ammonia, compared to conventional controls. This was associated with neuroinflammation and glial/microglial activation. Correlation network analysis in conventional mice showed significant linkages between systemic/neuroinflammation, intestinal microbiota, and ammonia. Specifically beneficial, autochthonous taxa were negatively linked with brain and systemic inflammation, ammonia, and with Staphylococcaceae, Lactobacillaceae, and Streptococcaceae. Enterobacteriaceae were positively linked with serum inflammatory cytokines.
Conclusion: Gut microbiota changes drive development of neuroinflammatory and systemic inflammatory responses in cirrhotic animals. (Hepatology 2016;64:1232-1248).
© 2016 by the American Association for the Study of Liver Diseases.
Conflict of interest statement
No competing interests exist for any author.
Figures












References
-
- Vilstrup H, Amodio P, Bajaj J, Cordoba J, Ferenci P, Mullen KD, Weissenborn K, et al. Hepatic encephalopathy in chronic liver disease: 2014 Practice Guideline by the American Association for the Study of Liver Diseases and the European Association for the Study of the Liver. Hepatology. 2014;60:715–735. - PubMed
-
- Shawcross DL, Davies NA, Williams R, Jalan R. Systemic inflammatory response exacerbates the neuropsychological effects of induced hyperammonemia in cirrhosis. J Hepatol. 2004;40:247–254. - PubMed
-
- Romero-Gomez M, Jover M, Galan JJ, Ruiz A. Gut ammonia production and its modulation. Metab Brain Dis. 2009;24:147–157. - PubMed
-
- Tenorio-Laranga J, Montoliu C, Urios A, Hernandez-Rabaza V, Ahabrach H, Garcia-Horsman JA, Felipo V. The expression levels of prolyl oligopeptidase responds not only to neuroinflammation but also to systemic inflammation upon liver failure in rat models and cirrhotic patients. J Neuroinflammation. 2015;12:183. - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

