Asn347 Glycosylation of Corticosteroid-binding Globulin Fine-tunes the Host Immune Response by Modulating Proteolysis by Pseudomonas aeruginosa and Neutrophil Elastase
- PMID: 27339896
- PMCID: PMC5016167
- DOI: 10.1074/jbc.M116.735258
Asn347 Glycosylation of Corticosteroid-binding Globulin Fine-tunes the Host Immune Response by Modulating Proteolysis by Pseudomonas aeruginosa and Neutrophil Elastase
Abstract
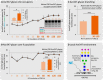
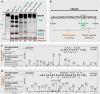
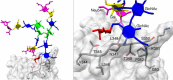
Corticosteroid-binding globulin (CBG) delivers anti-inflammatory cortisol to inflamed tissues upon elastase-based proteolysis of the exposed reactive center loop (RCL). However, the molecular mechanisms that regulate the RCL proteolysis by co-existing host and bacterial elastases in inflamed/infected tissues remain unknown. We document that RCL-localized Asn(347) glycosylation fine-tunes the RCL cleavage rate by human neutrophil elastase (NE) and Pseudomonas aeruginosa elastase (PAE) by different mechanisms. NE- and PAE-generated fragments of native and exoglycosidase-treated blood-derived CBG of healthy individuals were monitored by gel electrophoresis and LC-MS/MS to determine the cleavage site(s) and Asn(347) glycosylation as a function of digestion time. The site-specific (Val(344)-Thr(345)) and rapid (seconds to minutes) NE-based RCL proteolysis was significantly antagonized by several volume-enhancing Asn(347) glycan features (i.e. occupancy, triantennary GlcNAc branching, and α1,6-fucosylation) and augmented by Asn(347) NeuAc-type sialylation (all p < 0.05). In contrast, the inefficient (minutes to hours) PAE-based RCL cleavage, which occurred equally well at Thr(345)-Leu(346) and Asn(347)-Leu(348), was abolished by the presence of Asn(347) glycosylation but was enhanced by sialoglycans on neighboring CBG N-sites. Molecular dynamics simulations of various Asn(347) glycoforms of uncleaved CBG indicated that multiple Asn(347) glycan features are modulating the RCL digestion efficiencies by NE/PAE. Finally, high concentrations of cortisol showed weak bacteriostatic effects toward virulent P. aeruginosa, which may explain the low RCL potency of the abundantly secreted PAE during host infection. In conclusion, site-specific CBG N-glycosylation regulates the bioavailability of cortisol in inflamed environments by fine-tuning the RCL proteolysis by endogenous and exogenous elastases. This study offers new molecular insight into host- and pathogen-based manipulation of the human immune system.
Keywords: P. aeruginosa elastase; Pseudomonas aeruginosa (P. aeruginosa); corticosteroid-binding globulin; cortisol; glycoprotein; glycosylation; molecular dynamics; neutrophil elastase; proteolysis; reactive center loop.
© 2016 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures





References
-
- Siiteri P. K., Murai J. T., Hammond G. L., Nisker J. A., Raymoure W. J., and Kuhn R. W. (1982) The serum transport of steroid hormones. Recent Prog. Horm. Res. 38, 457–510 - PubMed
-
- Cameron A., Henley D., Carrell R., Zhou A., Clarke A., and Lightman S. (2010) Temperature-responsive release of cortisol from its binding globulin: a protein thermocouple. J. Clin. Endocrinol. Metab. 95, 4689–4695 - PubMed
-
- Khan M. S., Aden D., and Rosner W. (1984) Human corticosteroid binding globulin is secreted by a hepatoma-derived cell line. J. Steroid Biochem. 20, 677–678 - PubMed
-
- Hammond G. L., Smith C. L., and Underhill D. A. (1991) Molecular studies of corticosteroid binding globulin structure, biosynthesis and function. J. Steroid Biochem. Mol. Biol. 40, 755–762 - PubMed
-
- Hammond G. L., Smith C. L., Goping I. S., Underhill D. A., Harley M. J., Reventos J., Musto N. A., Gunsalus G. L., and Bardin C. W. (1987) Primary structure of human corticosteroid binding globulin, deduced from hepatic and pulmonary cDNAs, exhibits homology with serine protease inhibitors. Proc. Natl. Acad. Sci. U.S.A. 84, 5153–5157 - PMC - PubMed
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

