Myeloid-Specific Blockade of Notch Signaling Attenuates Choroidal Neovascularization through Compromised Macrophage Infiltration and Polarization in Mice
- PMID: 27339903
- PMCID: PMC4919651
- DOI: 10.1038/srep28617
Myeloid-Specific Blockade of Notch Signaling Attenuates Choroidal Neovascularization through Compromised Macrophage Infiltration and Polarization in Mice
Abstract
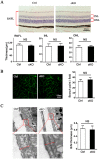
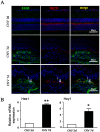
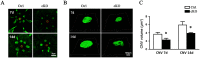
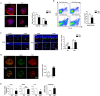
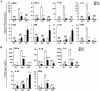
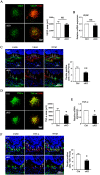
Macrophages have been recognized as an important inflammatory component in choroidal neovascularization (CNV). However, it is unclear how these cells are activated and polarized, how they affect angiogenesis and what the underlining mechanisms are during CNV. Notch signaling has been implicated in macrophage activation. Previously we have shown that inducible disruption of RBP-J, the critical transcription factor of Notch signaling, in adult mice results in enhanced CNV, but it is unclear what is the role of macrophage-specific Notch signaling in the development of CNV. In the current study, by using the myeloid specific RBP-J knockout mouse model combined with the laser-induced CNV model, we show that disruption of Notch signaling in macrophages displayed attenuated CNV growth, reduced macrophage infiltration and activation, and alleviated angiogenic response after laser induction. The inhibition of CNV occurred with reduced expression of VEGF and TNF-α in infiltrating inflammatory macrophages in myeloid specific RBP-J knockout mice. These changes might result in direct inhibition of EC lumen formation, as shown in an in vitro study. Therefore, clinical intervention of Notch signaling in CNV needs to pinpoint myeloid lineage to avoid the counteractive effects of global inhibition.
Figures

Similar articles
-
Myeloid-Specific Blockade of Notch Signaling by RBP-J Knockout Attenuates Spinal Cord Injury Accompanied by Compromised Inflammation Response in Mice.Mol Neurobiol. 2015 Dec;52(3):1378-1390. doi: 10.1007/s12035-014-8934-z. Epub 2014 Oct 26. Mol Neurobiol. 2015. PMID: 25344316
-
Differential role of tumor necrosis factor (TNF)-alpha receptors in the development of choroidal neovascularization.Invest Ophthalmol Vis Sci. 2010 Aug;51(8):3874-83. doi: 10.1167/iovs.09-5003. Epub 2010 Mar 24. Invest Ophthalmol Vis Sci. 2010. PMID: 20335614
-
Laser-induced choroidal neovascularization in mice attenuated by deficiency in the apelin-APJ system.Invest Ophthalmol Vis Sci. 2013 Jun 21;54(6):4321-9. doi: 10.1167/iovs.13-11611. Invest Ophthalmol Vis Sci. 2013. PMID: 23722395
-
The Role of Notch Signaling in Macrophages during Inflammation and Infection: Implication in Rheumatoid Arthritis?Cells. 2020 Jan 2;9(1):111. doi: 10.3390/cells9010111. Cells. 2020. PMID: 31906482 Free PMC article. Review.
-
The Notch signaling pathway regulates macrophage polarization in liver diseases.Int Immunopharmacol. 2021 Oct;99:107938. doi: 10.1016/j.intimp.2021.107938. Epub 2021 Aug 6. Int Immunopharmacol. 2021. PMID: 34371331 Review.
Cited by
-
Crosstalk Between H-Type Vascular Endothelial Cells and Macrophages: A Potential Regulator of Bone Homeostasis.J Inflamm Res. 2025 Feb 25;18:2743-2765. doi: 10.2147/JIR.S502604. eCollection 2025. J Inflamm Res. 2025. PMID: 40026304 Free PMC article. Review.
-
Transcriptome Analysis of Choroid and Retina From Tree Shrew With Choroidal Neovascularization Reveals Key Signaling Moieties.Front Genet. 2021 May 10;12:654955. doi: 10.3389/fgene.2021.654955. eCollection 2021. Front Genet. 2021. PMID: 34040635 Free PMC article.
-
Innate Immunity in Age-Related Macular Degeneration.Adv Exp Med Biol. 2021;1256:121-141. doi: 10.1007/978-3-030-66014-7_5. Adv Exp Med Biol. 2021. PMID: 33848000
-
Receptor-selective interleukin-4 mutein attenuates laser-induced choroidal neovascularization through the regulation of macrophage polarization in mice.Exp Ther Med. 2021 Dec;22(6):1367. doi: 10.3892/etm.2021.10801. Epub 2021 Sep 27. Exp Ther Med. 2021. PMID: 34659513 Free PMC article.
-
Lactate-activated macrophages induced aerobic glycolysis and epithelial-mesenchymal transition in breast cancer by regulation of CCL5-CCR5 axis: a positive metabolic feedback loop.Oncotarget. 2017 Nov 30;8(66):110426-110443. doi: 10.18632/oncotarget.22786. eCollection 2017 Dec 15. Oncotarget. 2017. PMID: 29299159 Free PMC article.
References
-
- Jager R. D., Mieler W. F. & Miller J. W. Age-related macular degeneration. The New England journal of medicine 358, 2606–2617 (2008). - PubMed
-
- Cherepanoff S., McMenamin P., Gillies M. C., Kettle E. & Sarks S. H. Bruch’s membrane and choroidal macrophages in early and advanced age-related macular degeneration. The British journal of ophthalmology 94, 918–925 (2010). - PubMed
-
- Apte R. S. Regulation of angiogenesis by macrophages. Advances in experimental medicine and biology 664, 15–19 (2010). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

