Hepatocyte nuclear factor 4α is required for cell differentiation and homeostasis in the adult mouse gastric epithelium
- PMID: 27340127
- PMCID: PMC5007292
- DOI: 10.1152/ajpgi.00195.2016
Hepatocyte nuclear factor 4α is required for cell differentiation and homeostasis in the adult mouse gastric epithelium
Abstract
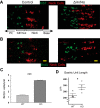
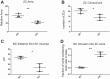
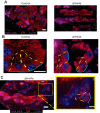
We have previously shown that the sequential transcription factors Xbp1→Mist1 (Bhlha15) govern the ultrastructural maturation of the secretory apparatus in enzyme-secreting zymogenic chief cells (ZCs) in the gastric unit. Here we sought to identify transcriptional regulators upstream of X-box binding protein 1 (XBP1) and MIST1. We used immunohistochemistry to characterize Hnf4α(flox/flox) adult mouse stomachs after tamoxifen-induced deletion of Hnf4α We used qRT-PCR, Western blotting, and chromatin immunoprecipitation to define the molecular interaction between hepatocyte nuclear factor 4 alpha (HNF4α) and Xbp1 in mouse stomach and human gastric cells. We show that HNF4α protein is expressed in pit (foveolar) cells, mucous neck cells, and zymogenic chief cells (ZCs) of the corpus gastric unit. Loss of HNF4α in adult mouse stomach led to reduced ZC size and ER content, phenocopying previously characterized effects of Xbp1 deletion. However, HNF4α(Δ/Δ) stomachs also exhibited additional phenotypes including increased proliferation in the isthmal stem cell zone and altered mucous neck cell migration, indicating a role of HNF4α in progenitor cells as well as in ZCs. HNF4α directly occupies the Xbp1 promoter locus in mouse stomach, and forced HNF4α expression increased abundance of XBP1 mRNA in human gastric cancer cells. Finally, as expected, loss of HNF4α caused decreased Xbp1 and Mist1 expression in mouse stomachs. We show that HNF4α regulates homeostatic proliferation in the gastric epithelium and is both necessary and sufficient for the upstream regulation of the Xbp1→Mist1 axis in maintenance of ZC secretory architecture.
Keywords: mucous neck cell; scaling factor; secretory cells.
Copyright © 2016 the American Physiological Society.
Figures




References
-
- Acosta-Alvear D, Zhou Y, Blais A, Tsikitis M, Lents NH, Arias C, Lennon CJ, Kluger Y, Dynlacht BD. XBP1 controls diverse cell type- and condition-specific transcriptional regulatory networks. Mol Cell 27: 53–66, 2007. - PubMed
-
- Babeu JP, Darsigny M, Lussier CR, Boudreau F. Hepatocyte nuclear factor 4alpha contributes to an intestinal epithelial phenotype in vitro and plays a partial role in mouse intestinal epithelium differentiation. Am J Physiol Gastrointest Liver Physiol 297: G124–G134, 2009. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

