Recruitment Into a Pediatric Continuous Glucose Monitoring RCT
- PMID: 27340247
- PMCID: PMC5375065
- DOI: 10.1177/1932296816656208
Recruitment Into a Pediatric Continuous Glucose Monitoring RCT
Abstract
Background: The purpose was to identify patient/family characteristics and recruitment process characteristics associated with the decision to participate in a 2-year continuous glucose monitoring (CGM) RCT for youth with type 1 diabetes and their families.
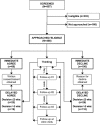
Method: Study staff approached patients who were conditionally eligible according to medical record review or referred by a provider. We categorized families according to participation decision (agree vs decline) and timing of decision (day of approach vs later ["thinkers"]).
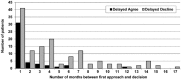
Results: Over 18 months, we approached 456 eligible patients; 19% agreed on the day of approach, 10% agreed later, 42% declined on the day of approach, and 30% declined later. Agreers were younger ( P = .002), had shorter diabetes duration ( P = .0003), had a lower insulin dose ( P = .02), checked blood glucose levels more often ( P = .002), and were more likely to use pump therapy ( P = .009) than decliners. Patients/families were more likely to agree in fall/winter (41%) than spring/summer (19%, P < .0001). Of decliners, 50% cited no interest in CGM as the reason for nonparticipation. Among thinkers, 49% of patients who made a decision within 2 weeks of being approached agreed; only 15% of thinkers who made a decision >2 weeks after being approached agreed to participate ( P < .0001).
Conclusions: Recruitment is a critical and often challenging phase of clinical trials. Recruitment to pediatric CGM studies may be especially challenging due to youths' reluctance to use CGM. These data provide an opportunity to better understand and possibly optimize recruitment into future pediatric CGM studies and other studies of advanced diabetes technologies.
Keywords: clinical research; continuous glucose monitoring; pediatrics; recruitment; type 1 diabetes.
Conflict of interest statement
Figures

Similar articles
-
Lessons learned from a pilot RCT of simultaneous versus delayed initiation of continuous glucose monitoring in children and adolescents with type 1 diabetes starting insulin pump therapy.J Diabetes Sci Technol. 2014 May;8(3):523-8. doi: 10.1177/1932296814524855. Epub 2014 Feb 27. J Diabetes Sci Technol. 2014. PMID: 24876616 Free PMC article. Clinical Trial.
-
The JDRF CCTN CGM TIME Trial: Timing of Initiation of continuous glucose Monitoring in Established pediatric type 1 diabetes: study protocol, recruitment and baseline characteristics.BMC Pediatr. 2014 Jul 18;14:183. doi: 10.1186/1471-2431-14-183. BMC Pediatr. 2014. PMID: 25034216 Free PMC article. Clinical Trial.
-
Diabetes technology and treatments in the paediatric age group.Int J Clin Pract Suppl. 2011 Feb;(170):76-82. doi: 10.1111/j.1742-1241.2010.02582.x. Int J Clin Pract Suppl. 2011. PMID: 21323816 Review.
-
Motivational Stage at Continuous Glucose Monitoring (CGM) Initiation in Pediatric Type 1 Diabetes Is Associated With Current Glycemic Control but Does Not Predict Future CGM Adherence or Glycemic Control.Can J Diabetes. 2021 Jul;45(5):466-472.e4. doi: 10.1016/j.jcjd.2021.04.004. Epub 2021 Apr 21. Can J Diabetes. 2021. PMID: 34176610 Clinical Trial.
-
Psychological Reactions Associated With Continuous Glucose Monitoring in Youth.J Diabetes Sci Technol. 2016 May 3;10(3):656-61. doi: 10.1177/1932296816638109. Print 2016 May. J Diabetes Sci Technol. 2016. PMID: 26969141 Free PMC article. Review.
Cited by
-
Distinct Patterns of Daily Glucose Variability by Pubertal Status in Youth With Type 1 Diabetes.Diabetes Care. 2020 Jan;43(1):22-28. doi: 10.2337/dc19-0083. Epub 2019 Jul 15. Diabetes Care. 2020. PMID: 31308020 Free PMC article.
-
Recruiting a representative sample in adherence research-The MALT multisite prospective cohort study experience.Pediatr Transplant. 2017 Dec;21(8):10.1111/petr.13067. doi: 10.1111/petr.13067. Epub 2017 Oct 6. Pediatr Transplant. 2017. PMID: 28984072 Free PMC article.
-
Parental Views of Facilitators and Barriers to Research Participation: Systematic Review.Pediatrics. 2023 Jan 1;151(1):e2022058067. doi: 10.1542/peds.2022-058067. Pediatrics. 2023. PMID: 36477217 Free PMC article.
-
Determination of Pubertal Status in Youths With Type 1 Diabetes Using Height Velocity and Trajectories.J Clin Endocrinol Metab. 2019 Jan 1;104(1):74-82. doi: 10.1210/jc.2018-01737. J Clin Endocrinol Metab. 2019. PMID: 30346541 Free PMC article.
References
-
- Caldwell PH, Murphy SB, Butow PN, Craig JC. Clinical trials in children. Lancet. 2004;364(9436):803-811. - PubMed
-
- Tait AR, Voepel-Lewis T, Malviya S. Participation of children in clinical research: factors that influence a parent’s decision to consent. Anesthesiology. 2003;99(4):819-825. - PubMed
-
- Caldwell PH, Butow PN, Craig JC. Parents’ attitudes to children’s participation in randomized controlled trials. J Pediatr. 2003;142(5):554-559. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

