High-dose beclometasone dipropionate/formoterol fumarate in fixed-dose combination for the treatment of asthma
- PMID: 27340255
- PMCID: PMC5933614
- DOI: 10.1177/1753465816654442
High-dose beclometasone dipropionate/formoterol fumarate in fixed-dose combination for the treatment of asthma
Abstract
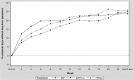
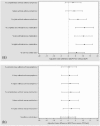
The high-strength formulation of extrafine beclometasone dipropionate/formoterol fumarate (BDP/Form) 200/6 µg has been developed to step up inhaled corticosteroid treatment, without increasing the dose of the bronchodilator, in patients who are not controlled with previous therapies. Two clinical studies have evaluated efficacy of high-strength BDP/Form as compared with another high-dose fixed combination and BDP monotherapy. Overall, data show that BDP/Form 200/6 μg improves lung function and has beneficial effects on symptoms, use of rescue medication and asthma control, with an acceptable safety profile comparable with that of high-dose fluticasone propionate/salmeterol. Therefore, BDP/Form 200/6 μg could be considered as an effective and safe treatment for patients with asthma who are not adequately controlled with high doses of inhaled corticosteroid monotherapy or medium doses of inhaled corticosteroid/long-acting β2-agonist combinations.
Keywords: asthma; beclometasone dipropionate/formoterol fumarate; high-dose fixed combination; inhaled corticosteroid; long-acting β2-agonist.
© The Author(s), 2016.
Conflict of interest statement
Figures

References
-
- Acerbi D., Brambilla G., Kottakis I. (2007) Advances in asthma and COPD management: delivering CFC-free inhaled therapy using Modulite technology. Pulm Pharmacol Ther 20: 290–303. - PubMed
-
- Allegra L., Cremonesi G., Girbino G., Ingrassia E., Marsico S., Nicolini G. (2012) Real-life prospective study on asthma control in Italy: cross-sectional phase results. Respir Med 106: 205–214. - PubMed
-
- Aubier M., Pieters W., Schlosser N., Steinmetz K. (1999) Salmeterol/fluticasone propionate (50/500 microg) in combination in a Diskus inhaler (Seretide) is effective and safe in the treatment of steroid-dependent asthma. Respir Med 93: 876–884. - PubMed
-
- Boyd G. (1995) Salmeterol xinafoate in asthmatic patients under consideration for maintenance oral corticosteroid therapy. UK Study Group. Eur Respir J 8: 1494–1498. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

