Resolution of Acute Inflammation and the Role of Resolvins in Immunity, Thrombosis, and Vascular Biology
- PMID: 27340271
- PMCID: PMC5260827
- DOI: 10.1161/CIRCRESAHA.116.307308
Resolution of Acute Inflammation and the Role of Resolvins in Immunity, Thrombosis, and Vascular Biology
Abstract
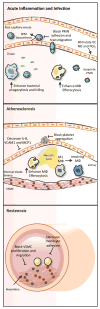
Acute inflammation is a host-protective response that is mounted in response to tissue injury and infection. Initiated and perpetuated by exogenous and endogenous mediators, acute inflammation must be resolved for tissue repair to proceed and for homeostasis to be restored. Resolution of inflammation is an actively regulated process governed by an array of mediators as diverse as those that initiate inflammation. Among these, resolvins have emerged as a genus of evolutionarily conserved proresolving mediators that act on specific cellular receptors to regulate leukocyte trafficking and blunt production of inflammatory mediators, while also promoting clearance of dead cells and tissue repair. Given that chronic unresolved inflammation is emerging as a central causative factor in the development of cardiovascular diseases, an understanding of the endogenous processes that govern normal resolution of acute inflammation is critical for determining why sterile maladaptive cardiovascular inflammation perpetuates. Here, we provide an overview of the process of resolution with a focus on the enzymatic biosynthesis and receptor-dependent actions of resolvins and related proresolving mediators in immunity, thrombosis, and vascular biology. We discuss how nutritional and current therapeutic approaches modulate resolution and propose that harnessing resolution concepts could potentially lead to the development of new approaches for treating chronic cardiovascular inflammation in a manner that is not host disruptive.
Keywords: cardiovascular diseases; fatty acids, omega-3; homeostasis; inflammation; lipids.
© 2016 American Heart Association, Inc.
Figures


References
-
- Shapiro MD, Fazio S. From Lipids to Inflammation: New Approaches to Reducing Atherosclerotic Risk. Circ Res. 2016;118:732–49. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

