Last line therapy with sorafenib in colorectal cancer: A retrospective analysis
- PMID: 27340356
- PMCID: PMC4911348
- DOI: 10.3748/wjg.v22.i23.5400
Last line therapy with sorafenib in colorectal cancer: A retrospective analysis
Abstract
Aim: To analyze the efficacy of last line sorafenib treatment in colorectal cancer patients.
Methods: All patients receiving chemotherapy for colorectal cancer in the outpatient clinic of the University of Mainz since 2006 were retrospectively analyzed for last line sorafenib exposure. Charts of identified patients were analyzed for clinic-pathological parameters, like data on gender, age, date of initial diagnosis, UICC stage, number and kind of the pre-therapies, therapy start and end of sorafenib, sorafenib mediated treatment cessation, side effects, response rates, time to progression and overall survival.
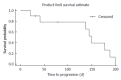
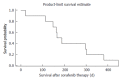
Results: Ten patients with a median of 3.0 prior chemotherapy lines had received a last line sorafenib therapy either alone (10%) or in combination with 5-fluorouracil derivates (90%). All patients suffered from colorectal cancer stage UICC 4 and were routinely seen in 2-wk intervals in the oncology outpatient clinic. Median duration of treatment was 142.0 d. At 8 wk 80% of patients showed stable disease but we did not observe any remissions. Median time to progression was 140.5 d (4.7 mo), while median overall survival reached 176.5 d. One patient ceased treatment due to side effects. Reason for treatment stop was bleeding complication in one case and non-specified sorafenib intolerance in another case. Due to the retrospective approach we did not further quantify side effects.
Conclusion: This retrospective analysis encourages further investigation of sorafenib in colorectal cancer last line therapy.
Keywords: Chemotherapy; Colorectal cancer; Last line; Regorafenib; Sorafenib.
Figures
References
-
- Greenlee RT, Murray T, Bolden S, Wingo PA. Cancer statistics, 2000. CA Cancer J Clin. 2000;50:7–33. - PubMed
-
- Weir HK, Thun MJ, Hankey BF, Ries LA, Howe HL, Wingo PA, Jemal A, Ward E, Anderson RN, Edwards BK. Annual report to the nation on the status of cancer, 1975-2000, featuring the uses of surveillance data for cancer prevention and control. J Natl Cancer Inst. 2003;95:1276–1299. - PubMed
-
- August DA, Ottow RT, Sugarbaker PH. Clinical perspective of human colorectal cancer metastasis. Cancer Metastasis Rev. 1984;3:303–324. - PubMed
-
- Douillard JY, Siena S, Cassidy J, Tabernero J, Burkes R, Barugel M, Humblet Y, Bodoky G, Cunningham D, Jassem J, et al. Randomized, phase III trial of panitumumab with infusional fluorouracil, leucovorin, and oxaliplatin (FOLFOX4) versus FOLFOX4 alone as first-line treatment in patients with previously untreated metastatic colorectal cancer: the PRIME study. J Clin Oncol. 2010;28:4697–4705. - PubMed
-
- Douillard JY, Siena S, Cassidy J, Tabernero J, Burkes R, Barugel M, Humblet Y, Bodoky G, Cunningham D, Jassem J, et al. Final results from PRIME: randomized phase III study of panitumumab with FOLFOX4 for first-line treatment of metastatic colorectal cancer. Ann Oncol. 2014;25:1346–1355. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical