Immunomodulatory Effects of Ambroxol on Airway Hyperresponsiveness and Inflammation
- PMID: 27340385
- PMCID: PMC4917400
- DOI: 10.4110/in.2016.16.3.165
Immunomodulatory Effects of Ambroxol on Airway Hyperresponsiveness and Inflammation
Abstract
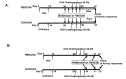
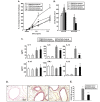
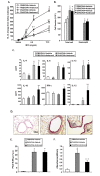
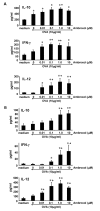
Ambroxol is used in COPD and asthma to increase mucociliary clearance and regulate surfactant levels, perhaps through anti-oxidant and anti-inflammatory activities. To determine the role and effect of ambroxol in an experimental model of asthma, BALB/c mice were sensitized to ovalbumin (OVA) followed by 3 days of challenge. Airway hyperresponsiveness (AHR), lung cell composition and histology, and cytokine and protein carbonyl levels in bronchoalveolar lavage (BAL) fluid were determined. Ambroxol was administered either before the first OVA challenge or was begun after the last allergen challenge. Cytokine production levels from lung mononuclear cells (Lung MNCs) or alveolar macrophages (AM) were also determined. Administration of ambroxol prior to challenge suppressed AHR, airway eosinophilia, goblet cell metaplasia, and reduced inflammation in subepithelial regions. When given after challenge, AHR was suppressed but without effects on eosinophil numbers. Levels of IL-5 and IL-13 in BAL fluid were decreased when the drug was given prior to challenge; when given after challenge, increased levels of IL-10 and IL-12 were detected. Decreased levels of protein carbonyls were detected in BAL fluid following ambroxol treatment after challenge. In vitro, ambroxol increased levels of IL-10, IFN-γ, and IL-12 from Lung MNCs and AM, whereas IL-4, IL-5, and IL-13 production was not altered. Taken together, ambroxol was effective in preventing AHR and airway inflammation through upregulation of Th1 cytokines and protection from oxidative stress in the airways.
Keywords: Airway hyperresponsiveness; Ambroxol; Eosinophils; Neutrophils.
Conflict of interest statement
Figures
Similar articles
-
Effects of combination therapy with montelukast and carbocysteine in allergen-induced airway hyperresponsiveness and airway inflammation.Br J Pharmacol. 2010 Jul;160(6):1399-407. doi: 10.1111/j.1476-5381.2010.00797.x. Br J Pharmacol. 2010. PMID: 20590630 Free PMC article.
-
Para-Bromophenacyl bromide alleviates airway hyperresponsiveness and modulates cytokines, IgE and eosinophil levels in ovalbumin-sensitized and -challenged mice.Int Immunopharmacol. 2004 Dec 15;4(13):1697-707. doi: 10.1016/j.intimp.2004.08.001. Int Immunopharmacol. 2004. PMID: 15454121
-
Syk activation in dendritic cells is essential for airway hyperresponsiveness and inflammation.Am J Respir Cell Mol Biol. 2006 Apr;34(4):426-33. doi: 10.1165/rcmb.2005-0298OC. Epub 2005 Dec 9. Am J Respir Cell Mol Biol. 2006. PMID: 16339999 Free PMC article.
-
Inhibition of early airway neutrophilia does not affect development of airway hyperresponsiveness.Am J Respir Cell Mol Biol. 2004 Jun;30(6):837-43. doi: 10.1165/rcmb.2003-0395OC. Epub 2004 Jan 23. Am J Respir Cell Mol Biol. 2004. PMID: 14742296
-
Cytokine and eosinophil responses in the lung, peripheral blood, and bone marrow compartments in a murine model of allergen-induced airways inflammation.Am J Respir Cell Mol Biol. 1997 May;16(5):510-20. doi: 10.1165/ajrcmb.16.5.9160833. Am J Respir Cell Mol Biol. 1997. PMID: 9160833
Cited by
-
Airway Redox Homeostasis and Inflammation Gone Awry: From Molecular Pathogenesis to Emerging Therapeutics in Respiratory Pathology.Int J Mol Sci. 2020 Dec 7;21(23):9317. doi: 10.3390/ijms21239317. Int J Mol Sci. 2020. PMID: 33297418 Free PMC article. Review.
-
Biological and antimicrobial properties of the association Ambroxol and a water-soluble viscous liquid as a vehicle for a tricalcium silicate-based sealer.J Mater Sci Mater Med. 2021 Nov 24;32(12):140. doi: 10.1007/s10856-021-06604-9. J Mater Sci Mater Med. 2021. PMID: 34817700 Free PMC article.
-
Safety and efficacy of inhalable ambroxol hydrochloride aerosol for adult patients with respiratory diseases: an open-label, single-arm, multicentre study.BMJ Open Respir Res. 2025 May 13;12(1):e002096. doi: 10.1136/bmjresp-2023-002096. BMJ Open Respir Res. 2025. PMID: 40360432 Free PMC article. Clinical Trial.
-
Characterization of differential patient profiles and therapeutic responses of pharmacy customers for four ambroxol formulations.BMC Pharmacol Toxicol. 2018 Jul 4;19(1):40. doi: 10.1186/s40360-018-0229-y. BMC Pharmacol Toxicol. 2018. PMID: 29973292 Free PMC article.
-
Ambroxol attenuates detrimental effect of LPS-induced glia-mediated neuroinflammation, oxidative stress, and cognitive dysfunction in mice brain.Front Immunol. 2025 Mar 6;16:1494114. doi: 10.3389/fimmu.2025.1494114. eCollection 2025. Front Immunol. 2025. PMID: 40114925 Free PMC article.
References
-
- Anandan C, Nurmatov U, van Schayck OC, Sheikh A. Is the prevalence of asthma declining? Systematic review of epidemiological studies. Allergy. 2010;65:152–167. - PubMed
-
- Lai HY, Rogers DF. Mucus hypersecretion in asthma: intracellular signaling pathways as targets for pharmacotherapy. Curr Opin Allergy Clin Immunol. 2010;10:67–76. - PubMed
-
- Pope SM, Brandt EB, Mishra A, Hogan SP, Zimmermann N, Matthaei KI, Foster PS, Rothenberg ME. IL-13 induces eosinophil recruitment into the lung by an IL-5- and eotaxin-dependent mechanism. J Allergy Clin Immunol. 2001;108:594–601. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

