Salvianolate Protects Hepatocytes from Oxidative Stress by Attenuating Mitochondrial Injury
- PMID: 27340417
- PMCID: PMC4909905
- DOI: 10.1155/2016/5408705
Salvianolate Protects Hepatocytes from Oxidative Stress by Attenuating Mitochondrial Injury
Abstract
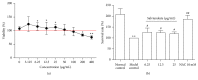
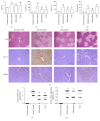
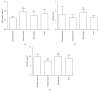
Salvianolate is widely used to treat angiocardiopathy in clinic in China, but its application in liver diseases remains unclear. Our study aims to investigate the effect of Salvianolate on rat hepatic injury by protecting hepatocyte mitochondria. To evaluate the effects of Salvianolate on injured hepatocytes, alpha mouse liver 12 (AML-12) cells were induced with hydrogen peroxide (H2O2) and treated with Salvianolate. Cell viability and MitoTracker Green for mitochondria and 5,5',6,6'-tetrachloro-1,1',3,3'-tetraethylbenzimidazole-carbocyanide iodine (JC-1) levels and cytochrome C (Cyto-C) expressions were detected in vitro. To identify the effect of Salvianolate on protecting against mitochondria injury, male Wistar rats were injected with carbon tetrachloride (CCl4) and treated with Salvianolate (40 mg·kg(-1)). Serum liver function, parameters for peroxidative damage, hematoxylin and eosin (H&E) staining, and transmission electron microscope (TEM) of hepatocyte mitochondria were assayed. Our results showed that Salvianolate effectively protected hepatocytes, increased mitochondria vitality, and decreased Cyto-C expressions in vitro. Besides, Salvianolate alleviated the liver function, attenuated the indicators of peroxidation, and relieved the mitochondria injury in vivo. In conclusion, Salvianolate is effective in protecting hepatocytes from injury in vitro and in vivo, and the mechanism might be related to its protective effect on hepatocyte mitochondria against oxidative stress.
Figures


Similar articles
-
[Salvianolate protects H9c2 cells from hypoxia/reoxygenation injury-induced apoptosis by attenuating mitochondrial DNA oxidative damage].Zhonghua Xin Xue Guan Bing Za Zhi. 2017 Jan 25;45(1):57-63. doi: 10.3760/cma.j.issn.0253-3758.2017.01.011. Zhonghua Xin Xue Guan Bing Za Zhi. 2017. PMID: 28100347 Chinese.
-
[The effects and related mechanism of salvianolate on rats with myocardial ischemia-reperfusion injury].Zhonghua Xin Xue Guan Bing Za Zhi. 2017 Dec 24;45(12):1072-1077. doi: 10.3760/cma.j.issn.0253-3758.2017.12.012. Zhonghua Xin Xue Guan Bing Za Zhi. 2017. PMID: 29325368 Chinese.
-
Salvianolic acid B protects hepatocytes from H2O2 injury by stabilizing the lysosomal membrane.World J Gastroenterol. 2017 Aug 7;23(29):5333-5344. doi: 10.3748/wjg.v23.i29.5333. World J Gastroenterol. 2017. PMID: 28839433 Free PMC article.
-
Protective effect of the glucagon-like peptide-1 analogue liraglutide on carbon tetrachloride-induced acute liver injury in mice.Biochem Biophys Res Commun. 2019 Jun 25;514(2):386-392. doi: 10.1016/j.bbrc.2019.04.160. Epub 2019 Apr 29. Biochem Biophys Res Commun. 2019. PMID: 31047638
-
Targeted Mitochondrial Delivery to Hepatocytes: A Review.J Clin Transl Hepatol. 2022 Apr 28;10(2):321-328. doi: 10.14218/JCTH.2021.00093. Epub 2021 Oct 19. J Clin Transl Hepatol. 2022. PMID: 35528979 Free PMC article. Review.
Cited by
-
Aqueous Extract of Davallia mariesii Attenuates 6-Hydroxydopamine-Induced Oxidative Damage and Apoptosis in B35 Cells Through Inhibition of Caspase Cascade and Activation of PI3K/AKT/GSK-3β Pathway.Nutrients. 2018 Oct 6;10(10):1449. doi: 10.3390/nu10101449. Nutrients. 2018. PMID: 30301204 Free PMC article.
-
Xueshuantong injection (lyophilized) combined with salvianolate lyophilized injection protects against focal cerebral ischemia/reperfusion injury in rats through attenuation of oxidative stress.Acta Pharmacol Sin. 2018 Jun;39(6):998-1011. doi: 10.1038/aps.2017.128. Epub 2017 Oct 12. Acta Pharmacol Sin. 2018. PMID: 29022576 Free PMC article.
-
Efficiently Capturing Mitochondria-Targeted Constituents with Hepatoprotective Activity from Medicinal Herbs.Oxid Med Cell Longev. 2019 Apr 9;2019:4353791. doi: 10.1155/2019/4353791. eCollection 2019. Oxid Med Cell Longev. 2019. PMID: 31093314 Free PMC article.
-
Hepatoprotective Effect of Pericarpium zanthoxyli Extract Is Mediated via Antagonism of Oxidative Stress.Evid Based Complement Alternat Med. 2020 Jul 9;2020:6761842. doi: 10.1155/2020/6761842. eCollection 2020. Evid Based Complement Alternat Med. 2020. PMID: 32695211 Free PMC article.
-
The Effects of Salvianolate Combined With Western Medicine on Diabetic Nephropathy: A Systematic Review and Meta-Analysis.Front Pharmacol. 2020 Jun 12;11:851. doi: 10.3389/fphar.2020.00851. eCollection 2020. Front Pharmacol. 2020. PMID: 32595500 Free PMC article.
References
-
- Rharass T., Lemcke H., Lantow M., Kuznetsov S. A., Weiss D. G., Panáková D. Ca2+-mediated mitochondrial reactive oxygen species metabolism augments Wnt/beta-catenin pathway activation to facilitate cell differentiation. The Journal of Biological Chemistry. 2014;289(40):27937–27951. doi: 10.1074/jbc.m114.573519. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

