Forces driving epithelial wound healing
- PMID: 27340423
- PMCID: PMC4915550
- DOI: 10.1038/nphys3040
Forces driving epithelial wound healing
Abstract
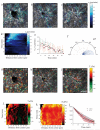
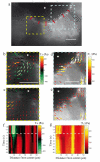
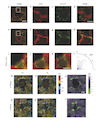
A fundamental feature of multicellular organisms is their ability to self-repair wounds through the movement of epithelial cells into the damaged area. This collective cellular movement is commonly attributed to a combination of cell crawling and "purse-string" contraction of a supracellular actomyosin ring. Here we show by direct experimental measurement that these two mechanisms are insufficient to explain force patterns observed during wound closure. At early stages of the process, leading actin protrusions generate traction forces that point away from the wound, showing that wound closure is initially driven by cell crawling. At later stages, we observed unanticipated patterns of traction forces pointing towards the wound. Such patterns have strong force components that are both radial and tangential to the wound. We show that these force components arise from tensions transmitted by a heterogeneous actomyosin ring to the underlying substrate through focal adhesions. The structural and mechanical organization reported here provides cells with a mechanism to close the wound by cooperatively compressing the underlying substrate.
Figures


References
-
- Cordeiro J. o. V., Jacinto A. n. The role of transcription-independent damage signals in the initiation of epithelial wound healing. Nat Rev Mol Cell Biol. 2013;14:249–262. - PubMed
-
- Arwert EN, Hoste E, Watt FM. Epithelial stem cells, wound healing and cancer. Nat Rev Cancer. 2012;12:170–180. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
