Aluminacyclopentanes in the synthesis of 3-substituted phospholanes and α,ω-bisphospholanes
- PMID: 27340436
- PMCID: PMC4902032
- DOI: 10.3762/bjoc.12.43
Aluminacyclopentanes in the synthesis of 3-substituted phospholanes and α,ω-bisphospholanes
Abstract
An efficient one-pot process for the synthesis of 3-substituted phospholanes and α,ω-bisphospholanes was developed. The method involves the replacement of aluminium in aluminacyclopentanes, prepared in situ by catalytic cycloalumination of α-olefins and α,ω-diolefins, by phosphorus atoms on treatment with dichlorophosphines (R'PCl2). Hydrogen peroxide oxidation and treatment with S8 of the synthesized phospholanes and α,ω-bisphospholanes afforded the corresponding 3-alkyl(aryl)-1-alkyl(phenyl)phospholane 1-oxides, 3-alkyl(aryl)-1-alkyl(phenyl)phospholane 1-sulfides, bisphospholane 1,1'-dioxides, and bisphospholane 1,1'-disulfides in nearly quantitative yields. The complexes LMo(CO)5 (L = 3-hexyl-1-phenylphospholane, 3-benzyl-1-methylphospholane, 1,2-bis(1-phenylphospholan-3-yl)ethane, and 1,6-bis(1-phenylphospholan-3-yl)hexane were prepared by the reaction of 3-substituted phospholanes and α,ω-bisphospholanes with molybdenum hexacarbonyl. The structure of the complexes was proved by multinuclear (1)H, (13)C, and (31)P spectroscopy.
Keywords: Dzhemilev reaction; aluminacyclopentanes; molybdenum complexes; phospholanes; zirconium complexes.
Figures





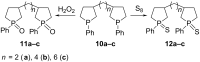
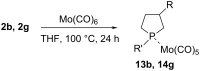

References
-
- Fagan P J, Nugent W A. J Am Chem Soc. 1988;110:2310–2312. doi: 10.1021/ja00215a057. - DOI
-
- Fagan P J, Nugent W A, Calabrese J C. J Am Chem Soc. 1994;116:1880–1889. doi: 10.1021/ja00084a031. - DOI
-
- Hydrio J, Gouygou M, Dallemer F, Daran J-C, Balavoine G G A. J Organomet Chem. 2000;595:261–267. doi: 10.1016/S0022-328X(99)00635-X. - DOI
-
- Zhou Y, Wang S, Chen C, Xi C. RSC Adv. 2015;5:71724–71727. doi: 10.1039/C5RA13818C. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
