Recent advances in N-heterocyclic carbene (NHC)-catalysed benzoin reactions
- PMID: 27340440
- PMCID: PMC4901930
- DOI: 10.3762/bjoc.12.47
Recent advances in N-heterocyclic carbene (NHC)-catalysed benzoin reactions
Erratum in
-
Correction: Recent advances in N-heterocyclic carbene (NHC)-catalysed benzoin reactions.Beilstein J Org Chem. 2016 Oct 4;12:2124. doi: 10.3762/bjoc.12.201. eCollection 2016. Beilstein J Org Chem. 2016. PMID: 27829918 Free PMC article.
Abstract
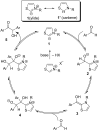
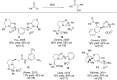
N-Heterocyclic carbenes (NHCs) have emerged as a powerful class of organocatalysts that mediate a variety of organic transformations. The Benzoin reaction constitutes one of the earliest known carbon-carbon bond-forming reactions catalysed by NHCs. The rapid growth of NHC catalysis in general has resulted in the development of a variety of benzoin and benzoin-type reactions. An overview of such NHC-catalysed benzoin reactions is presented.
Keywords: N-heterocyclic carbenes; acyloin reaction; benzoin reaction; organocatalysis; umpolung.
Figures
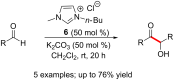


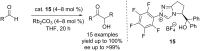
















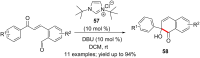


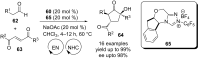





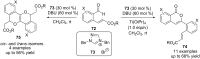
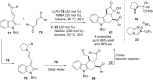
Similar articles
-
Correction: Recent advances in N-heterocyclic carbene (NHC)-catalysed benzoin reactions.Beilstein J Org Chem. 2016 Oct 4;12:2124. doi: 10.3762/bjoc.12.201. eCollection 2016. Beilstein J Org Chem. 2016. PMID: 27829918 Free PMC article.
-
Bifunctional N-Heterocyclic Carbenes Derived from l-Pyroglutamic Acid and Their Applications in Enantioselective Organocatalysis.Acc Chem Res. 2020 Mar 17;53(3):690-702. doi: 10.1021/acs.accounts.9b00635. Epub 2020 Mar 6. Acc Chem Res. 2020. PMID: 32142245 Review.
-
Remote Electronic Tuning of Chiral N-Heterocyclic Carbenes.Chem Rec. 2023 Jul;23(7):e202300103. doi: 10.1002/tcr.202300103. Epub 2023 May 31. Chem Rec. 2023. PMID: 37255345 Review.
-
The importance of four-membered NHCs in stabilizing Breslow intermediates on benzoin condensation pathway.J Comput Chem. 2023 Jan 30;44(3):346-354. doi: 10.1002/jcc.26935. Epub 2022 Jun 2. J Comput Chem. 2023. PMID: 35652523
-
N-Heterocyclic carbene (NHC) organocatalysis: from fundamentals to frontiers.Chem Soc Rev. 2025 Feb 3;54(3):1102-1124. doi: 10.1039/d4cs01179a. Chem Soc Rev. 2025. PMID: 39690964 Review.
Cited by
-
Direct α-Acylation of Alkenes via N-Heterocyclic Carbene, Sulfinate, and Photoredox Cooperative Triple Catalysis.J Am Chem Soc. 2021 Apr 7;143(13):4903-4909. doi: 10.1021/jacs.1c01022. Epub 2021 Mar 24. J Am Chem Soc. 2021. PMID: 33760603 Free PMC article.
-
An umpolung strategy for intermolecular [2 + 2 + 1] cycloaddition of aryl aldehydes and nitriles: a facile access to 2,4,5-trisubstituted oxazoles.Chem Sci. 2022 Jun 11;13(27):8080-8087. doi: 10.1039/d2sc00046f. eCollection 2022 Jul 13. Chem Sci. 2022. PMID: 35919435 Free PMC article.
-
Tunable Thiazolium Carbenes for Enantioselective Radical Three-Component Dicarbofunctionalizations.J Am Chem Soc. 2024 Dec 25;146(51):35199-35207. doi: 10.1021/jacs.4c11947. Epub 2024 Dec 10. J Am Chem Soc. 2024. PMID: 39656150 Free PMC article.
-
N-Heterocyclic-Carbene-Catalyzed Imine Umpolung for the Cross-Coupling of Quinoxalin-2-ones with Isatins.JACS Au. 2025 Jan 29;5(2):948-954. doi: 10.1021/jacsau.4c01166. eCollection 2025 Feb 24. JACS Au. 2025. PMID: 40017773 Free PMC article.
-
Ni/NHC-catalyzed C5-H alkylation and alkenylation of challenging furan(thiophene)-2-carboxaldehydes enabled by recyclable imine protecting group.Commun Chem. 2025 Aug 22;8(1):253. doi: 10.1038/s42004-025-01653-5. Commun Chem. 2025. PMID: 40846891 Free PMC article.
References
-
- Wöhler F, Liebig J. Ann Pharm. 1832;3:249. doi: 10.1002/jlac.18320030302. - DOI
-
- Ukai T, Tanaka R, Dokawa T. J Pharm Soc Jpn. 1943;63:296.
-
- Breslow R. J Am Chem Soc. 1958;80:3719. doi: 10.1021/ja01547a064. - DOI
-
- Igau A, Grutzmacher H, Baceiredo A, Bertrand G. J Am Chem Soc. 1988;110:6463. doi: 10.1021/ja00227a028. - DOI
-
- Arduengo A J, III, Harlow R L, Kline M. J Am Chem Soc. 1991;113:361. doi: 10.1021/ja00001a054. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
