Opportunities and challenges for direct C-H functionalization of piperazines
- PMID: 27340462
- PMCID: PMC4901899
- DOI: 10.3762/bjoc.12.70
Opportunities and challenges for direct C-H functionalization of piperazines
Abstract
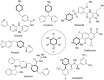
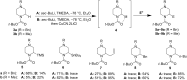
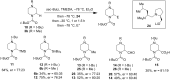
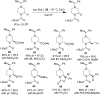
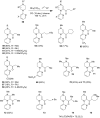
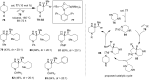
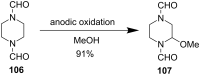
Piperazine ranks within the top three most utilized N-heterocyclic moieties in FDA-approved small-molecule pharmaceuticals. Herein we summarize the current synthetic methods available to perform C-H functionalization on piperazines in order to lend structural diversity to this privileged drug scaffold. Multiple approaches such as those involving α-lithiation trapping, transition-metal-catalyzed α-C-H functionalizations, and photoredox catalysis are discussed. We also highlight the difficulties experienced when successful methods for α-C-H functionalization of acyclic amines and saturated mono-nitrogen heterocyclic compounds (such as piperidines and pyrrolidines) were applied to piperazine substrates.
Keywords: C–H functionalization; heterocycle; photoredox catalysis; piperazine; α-lithiation.
Figures











Similar articles
-
Site-Selective C-H Alkylation of Piperazine Substrates via Organic Photoredox Catalysis.Org Lett. 2020 Jan 17;22(2):679-683. doi: 10.1021/acs.orglett.9b04456. Epub 2020 Jan 6. Org Lett. 2020. PMID: 31904980
-
Exploration of C-H Transformations of Aldehyde Hydrazones: Radical Strategies and Beyond.Acc Chem Res. 2018 Feb 20;51(2):484-495. doi: 10.1021/acs.accounts.7b00565. Epub 2018 Jan 23. Acc Chem Res. 2018. PMID: 29359909
-
Complex N-heterocycle synthesis via iron-catalyzed, direct C-H bond amination.Science. 2013 May 3;340(6132):591-5. doi: 10.1126/science.1233701. Science. 2013. PMID: 23641113
-
Recent developments in photoredox-catalyzed remote ortho and para C-H bond functionalizations.Beilstein J Org Chem. 2020 Feb 26;16:248-280. doi: 10.3762/bjoc.16.26. eCollection 2020. Beilstein J Org Chem. 2020. PMID: 32180843 Free PMC article. Review.
-
Palladium(0)-Catalyzed Benzylic C(sp3)-H Functionalization for the Concise Synthesis of Heterocycles and Its Applications.Chem Pharm Bull (Tokyo). 2017;65(5):409-425. doi: 10.1248/cpb.c16-00969. Chem Pharm Bull (Tokyo). 2017. PMID: 28458363 Review.
Cited by
-
Heterocyclic Merging of Stereochemically Diverse Chiral Piperazines and Morpholines with Indazoles.Chemistry. 2023 Oct 2;29(55):e202301888. doi: 10.1002/chem.202301888. Epub 2023 Aug 29. Chemistry. 2023. PMID: 37462979 Free PMC article.
-
Rapid functionalization of multiple C-H bonds in unprotected alicyclic amines.Nat Chem. 2020 Jun;12(6):545-550. doi: 10.1038/s41557-020-0438-z. Epub 2020 Mar 30. Nat Chem. 2020. PMID: 32231260 Free PMC article.
-
C-H Functionalization/activation in organic synthesis.Beilstein J Org Chem. 2016 Nov 3;12:2315-2316. doi: 10.3762/bjoc.12.224. eCollection 2016. Beilstein J Org Chem. 2016. PMID: 28144298 Free PMC article. No abstract available.
-
Recent progress toward the asymmetric synthesis of carbon-substituted piperazine pharmacophores and oxidative related heterocycles.RSC Med Chem. 2020 May 22;11(7):745-759. doi: 10.1039/d0md00053a. eCollection 2020 Jul 1. RSC Med Chem. 2020. PMID: 33479672 Free PMC article. Review.
-
DABCO bond cleavage for the synthesis of piperazine derivatives.RSC Adv. 2019 Nov 8;9(62):36386-36409. doi: 10.1039/c9ra07870c. eCollection 2019 Nov 4. RSC Adv. 2019. PMID: 35540608 Free PMC article. Review.
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
