Synthesis and in vitro cytotoxicity of acetylated 3-fluoro, 4-fluoro and 3,4-difluoro analogs of D-glucosamine and D-galactosamine
- PMID: 27340467
- PMCID: PMC4901990
- DOI: 10.3762/bjoc.12.75
Synthesis and in vitro cytotoxicity of acetylated 3-fluoro, 4-fluoro and 3,4-difluoro analogs of D-glucosamine and D-galactosamine
Abstract
Background: Derivatives of D-glucosamine and D-galactosamine represent an important family of the cell surface glycan components and their fluorinated analogs found use as metabolic inhibitors of complex glycan biosynthesis, or as probes for the study of protein-carbohydrate interactions. This work is focused on the synthesis of acetylated 3-deoxy-3-fluoro, 4-deoxy-4-fluoro and 3,4-dideoxy-3,4-difluoro analogs of D-glucosamine and D-galactosamine via 1,6-anhydrohexopyranose chemistry. Moreover, the cytotoxicity of the target compounds towards selected cancer cells is determined.
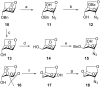
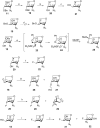
Results: Introduction of fluorine at C-3 was achieved by the reaction of 1,6-anhydro-2-azido-2-deoxy-4-O-benzyl-β-D-glucopyranose or its 4-fluoro analog with DAST. The retention of configuration in this reaction is discussed. Fluorine at C-4 was installed by the reaction of 1,6:2,3-dianhydro-β-D-talopyranose with DAST, or by fluoridolysis of 1,6:3,4-dianhydro-2-azido-β-D-galactopyranose with KHF2. The amino group was introduced and masked as an azide in the synthesis. The 1-O-deacetylated 3-fluoro and 4-fluoro analogs of acetylated D-galactosamine inhibited proliferation of the human prostate cancer cell line PC-3 more than cisplatin and 5-fluorouracil (IC50 28 ± 3 μM and 54 ± 5 μM, respectively).
Conclusion: A complete series of acetylated 3-fluoro, 4-fluoro and 3,4-difluoro analogs of D-glucosamine and D-galactosamine is now accessible by 1,6-anhydrohexopyranose chemistry. Intermediate fluorinated 1,6-anhydro-2-azido-hexopyranoses have potential as synthons in oligosaccharide assembly.
Keywords: amino sugars; cytotoxicity; fluorinated carbohydrates; fluorine; hexosamines.
Figures



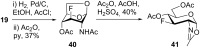

Similar articles
-
Synthesis of multiply fluorinated N-acetyl-D-glucosamine and D-galactosamine analogs via the corresponding deoxyfluorinated glucosazide and galactosazide phenyl thioglycosides.Beilstein J Org Chem. 2021 May 11;17:1086-1095. doi: 10.3762/bjoc.17.85. eCollection 2021. Beilstein J Org Chem. 2021. PMID: 34093878 Free PMC article.
-
Fluorinated carbohydrates as potential plasma membrane modifiers. Synthesis of 4- and 6-fluoro derivatives of 2-acetamido-2-deoxy-D-hexopyranoses.Carbohydr Res. 1990 May 1;198(2):205-21. doi: 10.1016/0008-6215(90)84293-4. Carbohydr Res. 1990. PMID: 2379186
-
Synthetic explorations towards 3-deoxy-3-fluoro derivatives of D-perosamine.Carbohydr Res. 2001 Aug 30;334(3):195-205. doi: 10.1016/s0008-6215(01)00188-4. Carbohydr Res. 2001. PMID: 11513826
-
Synthesis, structure, and biological applications of α-fluorinated β-amino acids and derivatives.Chem Biodivers. 2012 Nov;9(11):2410-41. doi: 10.1002/cbdv.201200307. Chem Biodivers. 2012. PMID: 23161626 Review.
-
Development of N-F fluorinating agents and their fluorinations: Historical perspective.Beilstein J Org Chem. 2021 Jul 27;17:1752-1813. doi: 10.3762/bjoc.17.123. eCollection 2021. Beilstein J Org Chem. 2021. PMID: 34386101 Free PMC article. Review.
Cited by
-
Skeletal rearrangement of 6,8-dioxabicyclo[3.2.1]octan-4-ols promoted by thionyl chloride or Appel conditions.Beilstein J Org Chem. 2024 Apr 16;20:823-829. doi: 10.3762/bjoc.20.74. eCollection 2024. Beilstein J Org Chem. 2024. PMID: 38655557 Free PMC article.
-
The Synthesis and Glycoside Formation of Polyfluorinated Carbohydrates.Chem Rev. 2022 Oct 26;122(20):15503-15602. doi: 10.1021/acs.chemrev.2c00086. Epub 2022 May 25. Chem Rev. 2022. PMID: 35613331 Free PMC article. Review.
-
Synthesis of multiply fluorinated N-acetyl-D-glucosamine and D-galactosamine analogs via the corresponding deoxyfluorinated glucosazide and galactosazide phenyl thioglycosides.Beilstein J Org Chem. 2021 May 11;17:1086-1095. doi: 10.3762/bjoc.17.85. eCollection 2021. Beilstein J Org Chem. 2021. PMID: 34093878 Free PMC article.
References
-
- Taylor M E, Drickamer K. Introduction to glycobiology. 3rd ed. Oxford, United Kingdom: Oxford University Press; 2011.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
