Next Generation Mesenchymal Stem Cell (MSC)-Based Cartilage Repair Using Scaffold-Free Tissue Engineered Constructs Generated with Synovial Mesenchymal Stem Cells
- PMID: 27340513
- PMCID: PMC4481383
- DOI: 10.1177/1947603515571002
Next Generation Mesenchymal Stem Cell (MSC)-Based Cartilage Repair Using Scaffold-Free Tissue Engineered Constructs Generated with Synovial Mesenchymal Stem Cells
Abstract
Because of its limited healing capacity, treatments for articular cartilage injuries are still challenging. Since the first report by Brittberg, autologous chondrocyte implantation has been extensively studied. Recently, as an alternative for chondrocyte-based therapy, mesenchymal stem cell-based therapy has received considerable research attention because of the relative ease in handling for tissue harvest, and subsequent cell expansion and differentiation. This review summarizes latest development of stem cell therapies in cartilage repair with special attention to scaffold-free approaches.
Keywords: animal models; articular cartilage; biomaterials; mesenchymal stem cells; synovial cells.
Conflict of interest statement
Figures




Similar articles
-
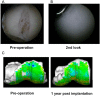
A novel scaffold-free mesenchymal stem cell-derived tissue engineered construct for articular cartilage restoration - From basic to clinic.Regen Ther. 2024 May 31;26:124-131. doi: 10.1016/j.reth.2024.05.007. eCollection 2024 Jun. Regen Ther. 2024. PMID: 38883147 Free PMC article. Review.
-
Scaffold-free tissue engineering for injured joint surface restoration.J Exp Orthop. 2018 Jan 5;5(1):2. doi: 10.1186/s40634-017-0118-0. J Exp Orthop. 2018. PMID: 29330730 Free PMC article. Review.
-
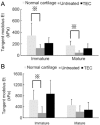
The influence of skeletal maturity on allogenic synovial mesenchymal stem cell-based repair of cartilage in a large animal model.Biomaterials. 2010 Nov;31(31):8004-11. doi: 10.1016/j.biomaterials.2010.07.017. Epub 2010 Jul 31. Biomaterials. 2010. PMID: 20674010
-
Chondrocytes, Mesenchymal Stem Cells, and Their Combination in Articular Cartilage Regenerative Medicine.Ann Biomed Eng. 2016 May;44(5):1325-54. doi: 10.1007/s10439-016-1575-9. Epub 2016 Mar 17. Ann Biomed Eng. 2016. PMID: 26987846 Review.
-
Chondrocyte and mesenchymal stem cell-based therapies for cartilage repair in osteoarthritis and related orthopaedic conditions.Maturitas. 2014 Jul;78(3):188-98. doi: 10.1016/j.maturitas.2014.04.017. Epub 2014 May 2. Maturitas. 2014. PMID: 24855933 Review.
Cited by
-
Editorial: Tissue Engineering and Cell Therapy for Cartilage Restoration.Front Cell Dev Biol. 2022 Jul 1;10:947588. doi: 10.3389/fcell.2022.947588. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35846365 Free PMC article. No abstract available.
-
Synthesis of photo-reactive poly (vinyl alcohol) and construction of scaffold-free cartilage like pellets in vitro.Regen Biomater. 2018 Jun;5(3):159-166. doi: 10.1093/rb/rby009. Epub 2018 May 3. Regen Biomater. 2018. PMID: 29942648 Free PMC article.
-
Perspective: Challenges Presented for Regeneration of Heterogeneous Musculoskeletal Tissues that Normally Develop in Unique Biomechanical Environments.Front Bioeng Biotechnol. 2021 Sep 28;9:760273. doi: 10.3389/fbioe.2021.760273. eCollection 2021. Front Bioeng Biotechnol. 2021. PMID: 34650964 Free PMC article. Review.
-
A novel scaffold-free mesenchymal stem cell-derived tissue engineered construct for articular cartilage restoration - From basic to clinic.Regen Ther. 2024 May 31;26:124-131. doi: 10.1016/j.reth.2024.05.007. eCollection 2024 Jun. Regen Ther. 2024. PMID: 38883147 Free PMC article. Review.
-
Stem Cells for Cartilage Regeneration: A Roadmap to the Clinic.Stem Cells Int. 2018 Apr 11;2018:7348560. doi: 10.1155/2018/7348560. eCollection 2018. Stem Cells Int. 2018. PMID: 29760735 Free PMC article. No abstract available.
References
-
- Hjelle K, Solheim E, Strand T, Muri R, Brittberg M. Articular cartilage defects in 1,000 knee arthroscopies. Arthroscopy. 2002;18:730-4. - PubMed
-
- Buckwalter JA. Articular cartilage injuries. Clin Orthop Relat Res. 2002;(402):21-37. - PubMed
-
- Hunziker EB. Articular cartilage repair: basic science and clinical progress. A review of the current status and prospects. Osteoarthritis Cartilage. 2002;10:432-63. - PubMed
-
- Mithoefer K, Williams RJ, 3rd, Warren RF, Potter HG, Spock CR, Jones EC, et al. The microfracture technique for the treatment of articular cartilage lesions in the knee. A prospective cohort study. J Bone Joint Surg Am. 2005;87:1911-20. - PubMed
-
- Nehrer S, Spector M, Minas T. Histologic analysis of tissue after failed cartilage repair procedures. Clin Orthop Relat Res. 1999;(365):149-62. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

