Progressive impairment of CaV1.1 function in the skeletal muscle of mice expressing a mutant type 1 Cu/Zn superoxide dismutase (G93A) linked to amyotrophic lateral sclerosis
- PMID: 27340545
- PMCID: PMC4918102
- DOI: 10.1186/s13395-016-0094-6
Progressive impairment of CaV1.1 function in the skeletal muscle of mice expressing a mutant type 1 Cu/Zn superoxide dismutase (G93A) linked to amyotrophic lateral sclerosis
Abstract
Background: Amyotrophic lateral sclerosis (ALS) is an adult-onset neurodegenerative disorder that is typically fatal within 3-5 years of diagnosis. While motoneuron death is the defining characteristic of ALS, the events that underlie its pathology are not restricted to the nervous system. In this regard, ALS muscle atrophies and weakens significantly before presentation of neurological symptoms. Since the skeletal muscle L-type Ca(2+) channel (CaV1.1) is a key regulator of both mass and force, we investigated whether CaV1.1 function is impaired in the muscle of two distinct mouse models carrying an ALS-linked mutation.
Methods: We recorded L-type currents, charge movements, and myoplasmic Ca(2+) transients from dissociated flexor digitorum brevis (FDB) fibers to assess CaV1.1 function in two mouse models expressing a type 1 Cu/Zn superoxide dismutase mutant (SOD1(G93A)).
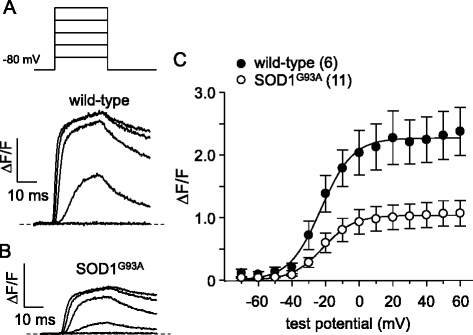
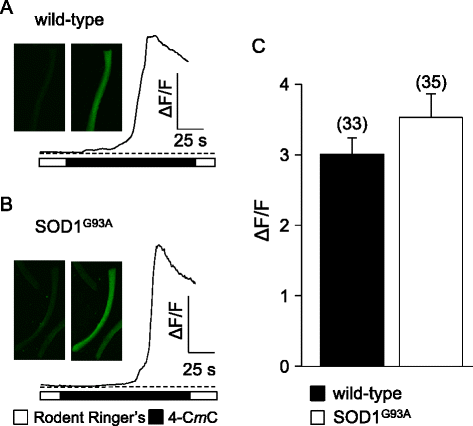
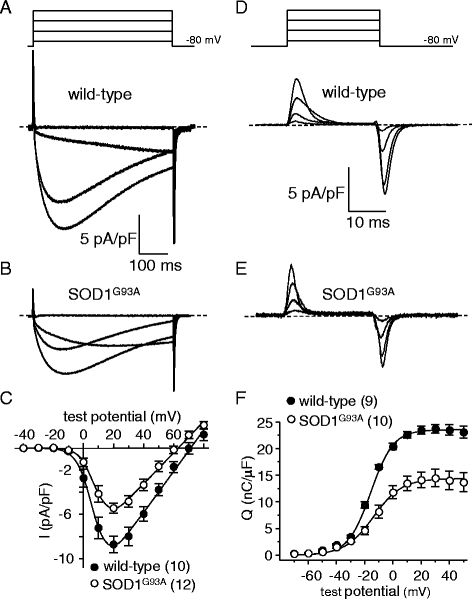
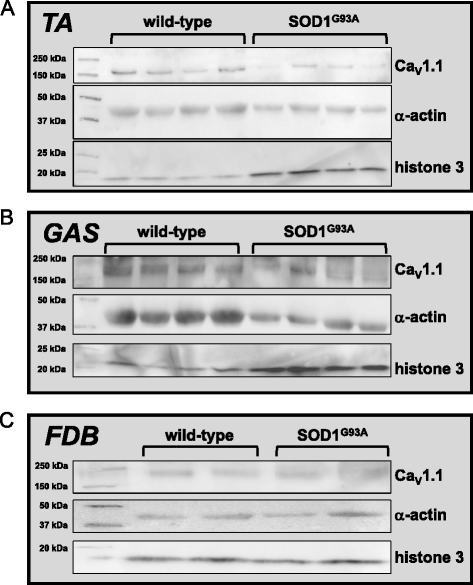
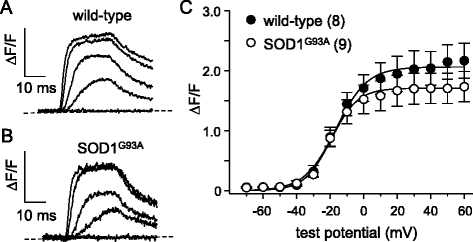
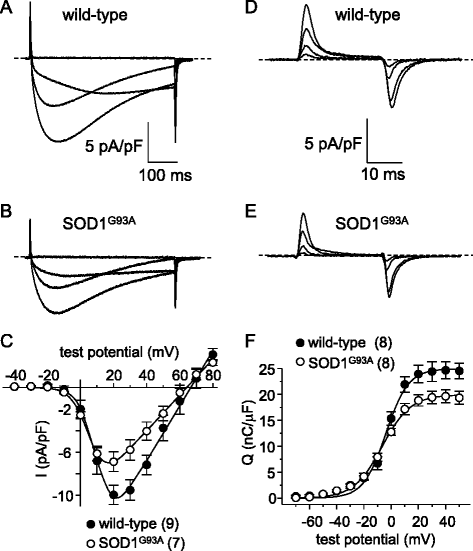
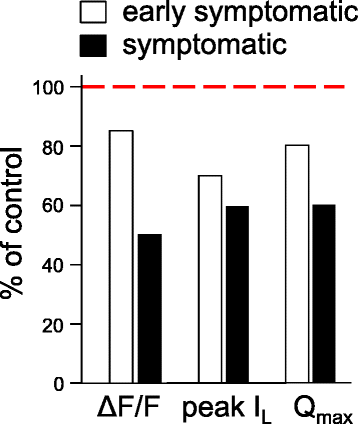
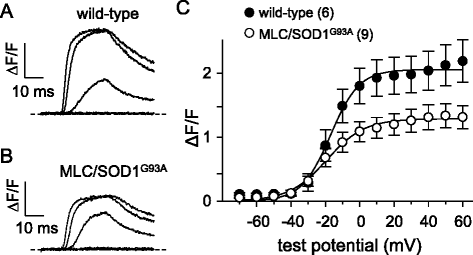
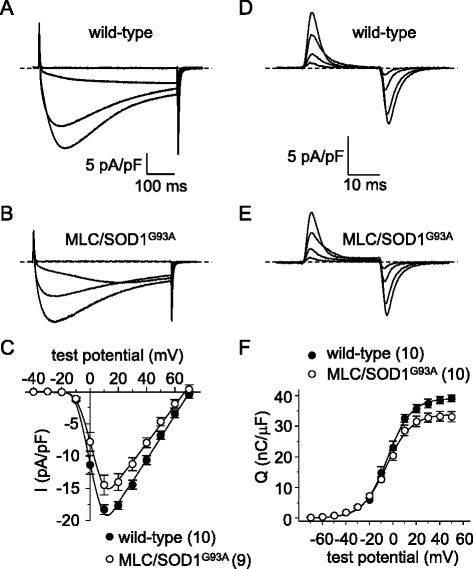
Results: In FDB fibers obtained from "symptomatic" global SOD1(G93A) mice, we observed a substantial reduction of SR Ca(2+) release in response to depolarization relative to fibers harvested from age-matched control mice. L-type current and charge movement were both reduced by ~40 % in symptomatic SOD1(G93A) fibers when compared to control fibers. Ca(2+) transients were not significantly reduced in similar experiments performed with FDB fibers obtained from "early-symptomatic" SOD1(G93A) mice, but L-type current and charge movement were decreased (~30 and ~20 %, respectively). Reductions in SR Ca(2+) release (~35 %), L-type current (~20 %), and charge movement (~15 %) were also observed in fibers obtained from another model where SOD1(G93A) expression was restricted to skeletal muscle.
Conclusions: We report reductions in EC coupling, L-type current density, and charge movement in FDB fibers obtained from symptomatic global SOD1(G93A) mice. Experiments performed with FDB fibers obtained from early-symptomatic SOD1(G93A) and skeletal muscle autonomous MLC/SOD1(G93A) mice support the idea that events occurring locally in the skeletal muscle contribute to the impairment of CaV1.1 function in ALS muscle independently of innervation status.
Keywords: ALS; Amyotrophic lateral sclerosis; CaV1.1; Excitation-contraction coupling; L-type; Neuromuscular disease; SOD1; Skeletal muscle.
Figures
Similar articles
-
Overexpression of Abeta is associated with acceleration of onset of motor impairment and superoxide dismutase 1 aggregation in an amyotrophic lateral sclerosis mouse model.Aging Cell. 2006 Apr;5(2):153-65. doi: 10.1111/j.1474-9726.2006.00200.x. Aging Cell. 2006. PMID: 16626394
-
The effect of peripheral nerve injury on disease progression in the SOD1(G93A) mouse model of amyotrophic lateral sclerosis.Neuroscience. 2005;130(4):897-910. doi: 10.1016/j.neuroscience.2004.09.069. Neuroscience. 2005. PMID: 15652988
-
Intact single muscle fibres from SOD1G93A amyotrophic lateral sclerosis mice display preserved specific force, fatigue resistance and training-like adaptations.J Physiol. 2019 Jun;597(12):3133-3146. doi: 10.1113/JP277456. Epub 2019 May 22. J Physiol. 2019. PMID: 31074054
-
Transgenic mouse model for familial amyotrophic lateral sclerosis with superoxide dismutase-1 mutation.Neuropathology. 2001 Mar;21(1):82-92. doi: 10.1046/j.1440-1789.2001.00361.x. Neuropathology. 2001. PMID: 11304046 Review.
-
Superoxide dismutase-1 mutation-related neurotoxicity in familial amyotrophic lateral sclerosis.Amyotroph Lateral Scler Other Motor Neuron Disord. 2000 Jun;1(3):143-61. doi: 10.1080/14660820050515151. Amyotroph Lateral Scler Other Motor Neuron Disord. 2000. PMID: 11464949 Review.
Cited by
-
Elucidating the Contribution of Skeletal Muscle Ion Channels to Amyotrophic Lateral Sclerosis in search of new therapeutic options.Sci Rep. 2019 Feb 28;9(1):3185. doi: 10.1038/s41598-019-39676-3. Sci Rep. 2019. PMID: 30816241 Free PMC article.
-
Aberrant evoked calcium signaling and nAChR cluster morphology in a SOD1 D90A hiPSC-derived neuromuscular model.Front Cell Dev Biol. 2024 Jun 20;12:1429759. doi: 10.3389/fcell.2024.1429759. eCollection 2024. Front Cell Dev Biol. 2024. PMID: 38966427 Free PMC article.
-
Optical Recording of Action Potential Initiation and Propagation in Mouse Skeletal Muscle Fibers.Biophys J. 2018 Dec 4;115(11):2127-2140. doi: 10.1016/j.bpj.2018.10.026. Epub 2018 Nov 3. Biophys J. 2018. PMID: 30448039 Free PMC article.
-
Swim Training Modulates Mouse Skeletal Muscle Energy Metabolism and Ameliorates Reduction in Grip Strength in a Mouse Model of Amyotrophic Lateral Sclerosis.Int J Mol Sci. 2019 Jan 9;20(2):233. doi: 10.3390/ijms20020233. Int J Mol Sci. 2019. PMID: 30634386 Free PMC article.
-
A mouse model of Huntington's disease shows altered ultrastructure of transverse tubules in skeletal muscle fibers.J Gen Physiol. 2021 Apr 5;153(4):e202012637. doi: 10.1085/jgp.202012637. J Gen Physiol. 2021. PMID: 33683318 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

