Cenderitide: structural requirements for the creation of a novel dual particulate guanylyl cyclase receptor agonist with renal-enhancing in vivo and ex vivo actions
- PMID: 27340557
- PMCID: PMC4853826
- DOI: 10.1093/ehjcvp/pvv040
Cenderitide: structural requirements for the creation of a novel dual particulate guanylyl cyclase receptor agonist with renal-enhancing in vivo and ex vivo actions
Abstract
Aims: Cenderitide is a novel dual natriuretic peptide (NP) receptor chimeric peptide activator, which targets the particulate guanylyl cyclase B (pGC-B) receptor and pGC-A unlike native NPs. Cenderitide was engineered to retain the anti-fibrotic properties of C-type natriuretic peptide (CNP)/pGC-B with renal-enhancing actions facilitated by fusion to the carboxyl terminus of Dendroaspis NP (DNP), a pGC-A agonist, to CNP. Here, we address significance of the DNP carboxyl terminus in dual pGC receptor activation and actions of cenderitide compared with CNP on renal function and cyclic guanosine monophosphate (cGMP) in vivo and ex vivo in normal canines.
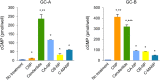
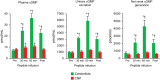
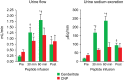
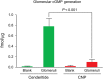
Methods and results: In vitro, only cenderitide and not CNP or three CNP-based variants was a potent dual pGC-A/pGC-B activator of cGMP production (from 5 to 237 pmol/mL) in human embryonic kidney (HEK) 293 cells overexpressing human pGC-A while in pGC-B overexpressing cells cenderitide increased cGMP production (from 4 to 321 pmol/mL) while the three CNP-based variants were weak agonists. Based upon our finding that the DNP carboxyl terminus is a key structural requirement for dual pGC-A/pGC-B activation, we defined in vivo the renal-enhancing actions of cenderitide compared with CNP. Cenderitide increased urinary cGMP excretion (from 989 to 5977 pmol/mL), net generation of renal cGMP (821-4124 pmol/min), natriuresis (12-242 μEq/min), and glomerular filtration rate (GFR) (37-51 mL/min) while CNP did not. We then demonstrated the transformation of CNP ex vivo into a renal cGMP-activating peptide which increased cGMP in freshly isolated glomeruli eight-fold greater than CNP.
Conclusion: The current study establishes that dual pGC-A and pGC-B activation with CNP requires the specific carboxyl terminus of DNP. In normal canines in vivo and in glomeruli ex vivo, the carboxyl terminus of DNP transforms CNP into a natriuretic and GFR-enhancing peptide. Future studies of cenderitide are warranted in cardiorenal disease states to explore its efficacy in overall cardiorenal homeostasis.
Keywords: C-type natriuretic peptide; CD-NP; Chimeric natriuretic peptide; canine.
Published on behalf of the European Society of Cardiology. All rights reserved. © The Author 2015. For permissions please email: journals.permissions@oup.com.
Figures
Comment in
-
Cenderitide: a multivalent designer-peptide-agonist of particulate guanylyl cyclase receptors with considerable therapeutic potential in cardiorenal disease states.Eur Heart J Cardiovasc Pharmacother. 2016 Apr;2(2):106-7. doi: 10.1093/ehjcvp/pvv043. Epub 2015 Dec 10. Eur Heart J Cardiovasc Pharmacother. 2016. PMID: 27533521 No abstract available.
Similar articles
-
C53: A novel particulate guanylyl cyclase B receptor activator that has sustained activity in vivo with anti-fibrotic actions in human cardiac and renal fibroblasts.J Mol Cell Cardiol. 2019 May;130:140-150. doi: 10.1016/j.yjmcc.2019.03.024. Epub 2019 Apr 4. J Mol Cell Cardiol. 2019. PMID: 30954448 Free PMC article.
-
A Human Study to Evaluate Safety, Tolerability, and Cyclic GMP Activating Properties of Cenderitide in Subjects With Stable Chronic Heart Failure.Clin Pharmacol Ther. 2018 Sep;104(3):546-552. doi: 10.1002/cpt.974. Epub 2018 Jan 11. Clin Pharmacol Ther. 2018. PMID: 29226471 Free PMC article. Clinical Trial.
-
Natriuretic peptide based therapeutics for heart failure: Cenderitide: A novel first-in-class designer natriuretic peptide.Int J Cardiol. 2019 Apr 15;281:166-171. doi: 10.1016/j.ijcard.2018.06.002. Epub 2018 Jun 3. Int J Cardiol. 2019. PMID: 29941213 Free PMC article. Review.
-
CRRL269: a novel designer and renal-enhancing pGC-A peptide activator.Am J Physiol Regul Integr Comp Physiol. 2018 Mar 1;314(3):R407-R414. doi: 10.1152/ajpregu.00286.2017. Epub 2017 Nov 29. Am J Physiol Regul Integr Comp Physiol. 2018. PMID: 29187381 Free PMC article.
-
Particulate Guanylyl Cyclase A/cGMP Signaling Pathway in the Kidney: Physiologic and Therapeutic Indications.Int J Mol Sci. 2018 Mar 27;19(4):1006. doi: 10.3390/ijms19041006. Int J Mol Sci. 2018. PMID: 29584705 Free PMC article. Review.
Cited by
-
Natriuretic Peptides in Heart Failure with Preserved Left Ventricular Ejection Fraction: From Molecular Evidences to Clinical Implications.Int J Mol Sci. 2019 May 28;20(11):2629. doi: 10.3390/ijms20112629. Int J Mol Sci. 2019. PMID: 31142058 Free PMC article. Review.
-
Cross talk on therapeutic strategies: natriuretic peptides and inhibiting neprilysin in hypertension management.Hypertens Res. 2025 Jan;48(1):284-300. doi: 10.1038/s41440-024-01989-w. Epub 2024 Nov 14. Hypertens Res. 2025. PMID: 39543415 Review.
-
Innovative Therapeutics: Designer Natriuretic Peptides.JACC Basic Transl Sci. 2016 Dec;1(7):557-567. doi: 10.1016/j.jacbts.2016.10.001. JACC Basic Transl Sci. 2016. PMID: 28497128 Free PMC article.
-
C53: A novel particulate guanylyl cyclase B receptor activator that has sustained activity in vivo with anti-fibrotic actions in human cardiac and renal fibroblasts.J Mol Cell Cardiol. 2019 May;130:140-150. doi: 10.1016/j.yjmcc.2019.03.024. Epub 2019 Apr 4. J Mol Cell Cardiol. 2019. PMID: 30954448 Free PMC article.
-
A Human Study to Evaluate Safety, Tolerability, and Cyclic GMP Activating Properties of Cenderitide in Subjects With Stable Chronic Heart Failure.Clin Pharmacol Ther. 2018 Sep;104(3):546-552. doi: 10.1002/cpt.974. Epub 2018 Jan 11. Clin Pharmacol Ther. 2018. PMID: 29226471 Free PMC article. Clinical Trial.
References
-
- Francis GS. From B-type natriuretic peptide to green mambas: the process of discovery. J Am Coll Cardiol 2008;52:69–70. - PubMed
-
- McKie PM, Sangaralingham SJ, Burnett JC Jr. CD-NP: an innovative designer natriuretic peptide activator of particulate guanylyl cyclase receptors for cardiorenal disease. Curr Heart Fail Rep 2010;7:93–99. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

