A Fusion Protein Consisting of the Vaccine Adjuvant Monophosphoryl Lipid A and the Allergen Ovalbumin Boosts Allergen-Specific Th1, Th2, and Th17 Responses In Vitro
- PMID: 27340679
- PMCID: PMC4908266
- DOI: 10.1155/2016/4156456
A Fusion Protein Consisting of the Vaccine Adjuvant Monophosphoryl Lipid A and the Allergen Ovalbumin Boosts Allergen-Specific Th1, Th2, and Th17 Responses In Vitro
Abstract
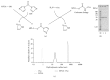
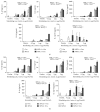
Background. The detoxified TLR4-ligand Monophosphoryl Lipid A (MPLA) is the first approved TLR-agonist used as adjuvant in licensed vaccines but has not yet been explored as part of conjugated vaccines. Objective. To investigate the immune-modulating properties of a fusion protein consisting of MPLA and Ovalbumin (MPLA : Ova). Results. MPLA and Ova were chemically coupled by stable carbamate linkage. MPLA : Ova was highly pure without detectable product-related impurities by either noncoupled MPLA or Ova. Light scattering analysis revealed MPLA : Ova to be aggregated. Stimulation of mDC and mDC : DO11.10 CD4(+) TC cocultures showed a stronger activation of both mDC and Ova-specific DO11.10 CD4(+) TC by MPLA : Ova compared to the mixture of both components. MPLA : Ova induced both strong proinflammatory (IL-1β, IL-6, and TNF-α) and anti-inflammatory (IL-10) cytokine responses from mDCs while also boosting allergen-specific Th1, Th2, and Th17 cytokine secretion. Conclusion. Conjugation of MPLA and antigen enhanced the immune response compared to the mixture of both components. Due to the nonbiased boost of Ova-specific Th2 and Th17 responses while also inducing Th1 responses, this fusion protein may not be a suitable vaccine candidate for allergy treatment but may hold potential for the treatment of other diseases that require a strong stimulation of the host's immune system (e.g., cancer).
Figures

Similar articles
-
MPLA shows attenuated pro-inflammatory properties and diminished capacity to activate mast cells in comparison with LPS.Allergy. 2015 Oct;70(10):1259-68. doi: 10.1111/all.12675. Epub 2015 Jul 8. Allergy. 2015. PMID: 26081583
-
Prophylactic vaccination with adjuvant monophosphoryl lipid a prevents Th2-mediated murine asthmatic responses.J Asthma. 2013 May;50(4):327-33. doi: 10.3109/02770903.2013.769268. Epub 2013 Feb 20. J Asthma. 2013. PMID: 23343407
-
A fusion protein of flagellin and ovalbumin suppresses the TH2 response and prevents murine intestinal allergy.J Allergy Clin Immunol. 2011 Dec;128(6):1340-1348.e12. doi: 10.1016/j.jaci.2011.07.036. Epub 2011 Aug 26. J Allergy Clin Immunol. 2011. PMID: 21872305
-
Allergy vaccines--new approaches to an old concept.Expert Opin Biol Ther. 2004 Sep;4(9):1473-81. doi: 10.1517/14712598.4.9.1473. Expert Opin Biol Ther. 2004. PMID: 15335314 Review.
-
In vivo direction of CD4 T cells to Th1 and Th2-like patterns of cytokine synthesis.Adv Exp Med Biol. 1996;409:309-16. doi: 10.1007/978-1-4615-5855-2_44. Adv Exp Med Biol. 1996. PMID: 9095259 Review.
Cited by
-
Spatio-temporal delivery of both intra- and extracellular toll-like receptor agonists for enhancing antigen-specific immune responses.Acta Pharm Sin B. 2022 Dec;12(12):4486-4500. doi: 10.1016/j.apsb.2022.05.032. Epub 2022 Jun 3. Acta Pharm Sin B. 2022. PMID: 36561992 Free PMC article.
-
Immunomodulatory Response of Toll-like Receptor Ligand-Peptide Conjugates in Food Allergy.ACS Chem Biol. 2021 Nov 19;16(11):2651-2664. doi: 10.1021/acschembio.1c00765. Epub 2021 Nov 11. ACS Chem Biol. 2021. PMID: 34761908 Free PMC article.
-
Adjuvants for allergy immunotherapeutics.Hum Vaccin Immunother. 2017 Oct 3;13(10):2416-2427. doi: 10.1080/21645515.2017.1348447. Hum Vaccin Immunother. 2017. PMID: 28825867 Free PMC article. Review.
-
Nanomedicine-mediated alteration of the pharmacokinetic profile of small molecule cancer immunotherapeutics.Acta Pharmacol Sin. 2020 Jul;41(7):881-894. doi: 10.1038/s41401-020-0425-3. Epub 2020 May 25. Acta Pharmacol Sin. 2020. PMID: 32451411 Free PMC article. Review.
-
Toll-like Receptor Agonist Conjugation: A Chemical Perspective.Bioconjug Chem. 2018 Mar 21;29(3):587-603. doi: 10.1021/acs.bioconjchem.7b00808. Epub 2018 Feb 16. Bioconjug Chem. 2018. PMID: 29378134 Free PMC article. Review.
References
-
- Justicia J. L., Cardona V., Guardia P., et al. Validation of the first treatment-specific questionnaire for the assessment of patient satisfaction with allergen-specific immunotherapy in allergic patients: the ESPIA questionnaire. Journal of Allergy and Clinical Immunology. 2013;131(6):1539–1546.e2. doi: 10.1016/j.jaci.2012.11.049. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

