Local CXCR4 Upregulation in the Injured Arterial Wall Contributes to Intimal Hyperplasia
- PMID: 27340942
- PMCID: PMC5113668
- DOI: 10.1002/stem.2442
Local CXCR4 Upregulation in the Injured Arterial Wall Contributes to Intimal Hyperplasia
Abstract
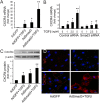
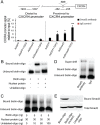
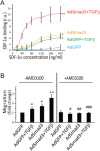
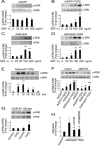
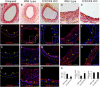
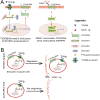
CXCR4 is a stem/progenitor cell surface receptor specific for the cytokine stromal cell-derived factor-1 (SDF-1α). There is evidence that bone marrow-derived CXCR4-expressing cells contribute to intimal hyperplasia (IH) by homing to the arterial subintima which is enriched with SDF-1α. We have previously found that transforming growth factor-β (TGFβ) and its signaling protein Smad3 are both upregulated following arterial injury and that TGFβ/Smad3 enhances the expression of CXCR4 in vascular smooth muscle cells (SMCs). It remains unknown, however, whether locally induced CXCR4 expression in SM22 expressing vascular SMCs plays a role in neointima formation. Here, we investigated whether elevated TGFβ/Smad3 signaling leads to the induction of CXCR4 expression locally in the injured arterial wall, thereby contributing to IH. We found prominent CXCR4 upregulation (mRNA, 60-fold; protein, 4-fold) in TGFβ-treated, Smad3-expressing SMCs. Chromatin immunoprecipitation assays revealed a specific association of the transcription factor Smad3 with the CXCR4 promoter. TGFβ/Smad3 treatment also markedly enhanced SDF-1α-induced ERK1/2 phosphorylation as well as SMC migration in a CXCR4-dependent manner. Adenoviral expression of Smad3 in balloon-injured rat carotid arteries increased local CXCR4 levels and enhanced IH, whereas SMC-specific depletion of CXCR4 in the wire-injured mouse femoral arterial wall produced a 60% reduction in IH. Our results provide the first evidence that upregulation of TGFβ/Smad3 in injured arteries induces local SMC CXCR4 expression and cell migration, and consequently IH. The Smad3/CXCR4 pathway may provide a potential target for therapeutic interventions to prevent restenosis. Stem Cells 2016;34:2744-2757.
Keywords: CXCR4/SDF-1α; TGFβ/Smad3; intimal hyperplasia; smooth muscle cell migration; smooth muscle cell specific CXCR4 knockout.
© 2016 The Authors STEM CELLS published by Wiley Periodicals, Inc. on behalf of AlphaMed Press.
Figures

Similar articles
-
TGF-β/Smad3 inhibit vascular smooth muscle cell apoptosis through an autocrine signaling mechanism involving VEGF-A.Cell Death Dis. 2014 Jul 10;5(7):e1317. doi: 10.1038/cddis.2014.282. Cell Death Dis. 2014. PMID: 25010983 Free PMC article.
-
Targeted deletion of PTEN in smooth muscle cells results in vascular remodeling and recruitment of progenitor cells through induction of stromal cell-derived factor-1alpha.Circ Res. 2008 May 9;102(9):1036-45. doi: 10.1161/CIRCRESAHA.107.169896. Epub 2008 Mar 13. Circ Res. 2008. PMID: 18340011
-
FAM3A promotes vascular smooth muscle cell proliferation and migration and exacerbates neointima formation in rat artery after balloon injury.J Mol Cell Cardiol. 2014 Sep;74:173-82. doi: 10.1016/j.yjmcc.2014.05.011. Epub 2014 May 23. J Mol Cell Cardiol. 2014. PMID: 24857820
-
TGFβ, smooth muscle cells and coronary artery disease: a review.Cell Signal. 2019 Jan;53:90-101. doi: 10.1016/j.cellsig.2018.09.004. Epub 2018 Sep 15. Cell Signal. 2019. PMID: 30227237 Free PMC article. Review.
-
The role of progenitor cells in the development of intimal hyperplasia.J Vasc Surg. 2009 Feb;49(2):502-10. doi: 10.1016/j.jvs.2008.07.060. Epub 2008 Oct 22. J Vasc Surg. 2009. PMID: 18945574 Free PMC article. Review.
Cited by
-
Differential gene expression and miRNA regulatory network in coronary slow flow.Sci Rep. 2024 Apr 10;14(1):8419. doi: 10.1038/s41598-024-58745-w. Sci Rep. 2024. PMID: 38600259 Free PMC article.
-
MEK1/2 inhibitor inhibits neointima formation by activating miR-126-3p/ C-X-C motif chemokine ligand 12 (CXCL12)/C-X-C motif chemokine receptor 4 (CXCR4) axis.Bioengineered. 2022 Apr;13(4):11214-11227. doi: 10.1080/21655979.2022.2063496. Bioengineered. 2022. PMID: 35485167 Free PMC article.
-
A Role for Polo-Like Kinase 4 in Vascular Fibroblast Cell-Type Transition.JACC Basic Transl Sci. 2021 Mar 22;6(3):257-283. doi: 10.1016/j.jacbts.2020.12.015. eCollection 2021 Mar. JACC Basic Transl Sci. 2021. PMID: 33778212 Free PMC article.
-
Smad2 inhibition of MET transcription potentiates human vascular smooth muscle cell apoptosis.Atheroscler Plus. 2021 Oct;44:31-42. doi: 10.1016/j.athplu.2021.08.005. Epub 2021 Aug 21. Atheroscler Plus. 2021. PMID: 35445204 Free PMC article.
-
Imaging of chemokine receptor CXCR4 expression in culprit and nonculprit coronary atherosclerotic plaque using motion-corrected [68Ga]pentixafor PET/CT.Eur J Nucl Med Mol Imaging. 2018 Oct;45(11):1934-1944. doi: 10.1007/s00259-018-4076-2. Epub 2018 Jul 3. Eur J Nucl Med Mol Imaging. 2018. PMID: 29967943 Free PMC article.
References
-
- Mills B, Robb T, Larson DF. Intimal hyperplasia: slow but deadly. Perfusion 2012;27:520–528. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

