The coming of age of the mitochondria-ER contact: a matter of thickness
- PMID: 27341186
- PMCID: PMC5072433
- DOI: 10.1038/cdd.2016.52
The coming of age of the mitochondria-ER contact: a matter of thickness
Abstract
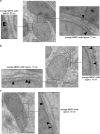
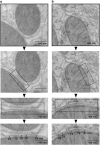
The sites of near-contact between the mitochondrion and the endoplasmic reticulum (ER) have earned a lot of attention due to their key role in the maintenance of lipid and calcium (Ca(2+)) homeostasis, in the initiation of autophagy and mitochondrial division, and in sensing metabolic shifts. At these sites, typically called MAMs (mitochondria-associated ER membranes) or MERCs (mitochondria-ER contacts), the organelles juxtapose at a distance that can range from ~10 to ~50 nm. The multifunctional role of this subcellular compartment is puzzling; further, recent studies have shown that mitochondria-ER contacts are highly plastic structures that remodel upon metabolic transitions and that their activity in controlling lipid homeostasis could be involved in Alzheimer's disease pathogenesis. This review aims at integrating the functions of this subcellular compartment to its most characterizing and unexplored structural parameter, their 'thickness': that is, the width of the cleft that separates the cytosolic face of the outer mitochondrial membrane from that of the ER. We describe and discuss the reasons why the thickness of a MERC should be considered a regulated structural parameter of the cell that defines and controls its function. Further, we propose a MERC classification that will help organize the expanding field of MERCs biology and of their role in cell physiology and human disease.
Figures


References
-
- Montisano DF, Cascarano J, Pickett CB, James TW. Association between mitochondria and rough endoplasmic reticulum in rat liver. Anat Rec 1982; 203: 441–450. - PubMed
-
- Pickett CB, Montisano D, Eisner D, Cascarano J. The physical association between rat liver mitochondria and rough endoplasmic reticulum. I. Isolation, electron microscopic examination and sedimentation equilibrium centrifugation analyses of rough endoplasmic reticulum-mitochondrial complexes. Exp Cell Res 1980; 128: 343–352. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

