C-Terminal Tyrosine Residue Modifications Modulate the Protective Phosphorylation of Serine 129 of α-Synuclein in a Yeast Model of Parkinson's Disease
- PMID: 27341336
- PMCID: PMC4920419
- DOI: 10.1371/journal.pgen.1006098
C-Terminal Tyrosine Residue Modifications Modulate the Protective Phosphorylation of Serine 129 of α-Synuclein in a Yeast Model of Parkinson's Disease
Abstract
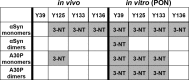
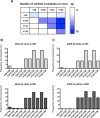
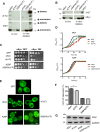
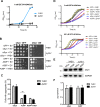
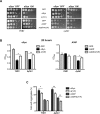
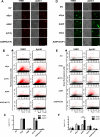
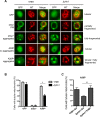
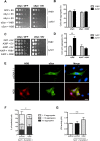
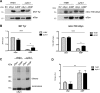
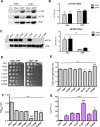
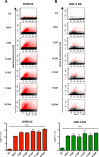
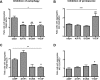
Parkinson´s disease (PD) is characterized by the presence of proteinaceous inclusions called Lewy bodies that are mainly composed of α-synuclein (αSyn). Elevated levels of oxidative or nitrative stresses have been implicated in αSyn related toxicity. Phosphorylation of αSyn on serine 129 (S129) modulates autophagic clearance of inclusions and is prominently found in Lewy bodies. The neighboring tyrosine residues Y125, Y133 and Y136 are phosphorylation and nitration sites. Using a yeast model of PD, we found that Y133 is required for protective S129 phosphorylation and for S129-independent proteasome clearance. αSyn can be nitrated and form stable covalent dimers originating from covalent crosslinking of two tyrosine residues. Nitrated tyrosine residues, but not di-tyrosine-crosslinked dimers, contributed to αSyn cytotoxicity and aggregation. Analysis of tyrosine residues involved in nitration and crosslinking revealed that the C-terminus, rather than the N-terminus of αSyn, is modified by nitration and di-tyrosine formation. The nitration level of wild-type αSyn was higher compared to that of A30P mutant that is non-toxic in yeast. A30P formed more dimers than wild-type αSyn, suggesting that dimer formation represents a cellular detoxification pathway in yeast. Deletion of the yeast flavohemoglobin gene YHB1 resulted in an increase of cellular nitrative stress and cytotoxicity leading to enhanced aggregation of A30P αSyn. Yhb1 protected yeast from A30P-induced mitochondrial fragmentation and peroxynitrite-induced nitrative stress. Strikingly, overexpression of neuroglobin, the human homolog of YHB1, protected against αSyn inclusion formation in mammalian cells. In total, our data suggest that C-terminal Y133 plays a major role in αSyn aggregate clearance by supporting the protective S129 phosphorylation for autophagy and by promoting proteasome clearance. C-terminal tyrosine nitration increases pathogenicity and can only be partially detoxified by αSyn di-tyrosine dimers. Our findings uncover a complex interplay between S129 phosphorylation and C-terminal tyrosine modifications of αSyn that likely participates in PD pathology.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Similar articles
-
Tyrosine 136 phosphorylation of α-synuclein aggregates in the Lewy body dementia brain: involvement of serine 129 phosphorylation by casein kinase 2.Acta Neuropathol Commun. 2021 Nov 12;9(1):182. doi: 10.1186/s40478-021-01281-9. Acta Neuropathol Commun. 2021. PMID: 34772466 Free PMC article.
-
α-Synuclein Decreases the Abundance of Proteasome Subunits and Alters Ubiquitin Conjugates in Yeast.Cells. 2021 Aug 28;10(9):2229. doi: 10.3390/cells10092229. Cells. 2021. PMID: 34571878 Free PMC article.
-
Aggregate clearance of α-synuclein in Saccharomyces cerevisiae depends more on autophagosome and vacuole function than on the proteasome.J Biol Chem. 2012 Aug 10;287(33):27567-79. doi: 10.1074/jbc.M112.361865. Epub 2012 Jun 21. J Biol Chem. 2012. PMID: 22722939 Free PMC article.
-
Posttranslational Modifications and Clearing of α-Synuclein Aggregates in Yeast.Biomolecules. 2015 Apr 23;5(2):617-34. doi: 10.3390/biom5020617. Biomolecules. 2015. PMID: 25915624 Free PMC article. Review.
-
Ubiquitination of alpha-synuclein and autophagy in Parkinson's disease.Autophagy. 2008 Apr;4(3):372-4. doi: 10.4161/auto.5604. Epub 2008 Jan 18. Autophagy. 2008. PMID: 18216494 Review.
Cited by
-
Parkinson's Disease: From Pathogenesis to Pharmacogenomics.Int J Mol Sci. 2017 Mar 4;18(3):551. doi: 10.3390/ijms18030551. Int J Mol Sci. 2017. PMID: 28273839 Free PMC article. Review.
-
In Search of Effective Treatments Targeting α-Synuclein Toxicity in Synucleinopathies: Pros and Cons.Front Cell Dev Biol. 2020 Sep 4;8:559791. doi: 10.3389/fcell.2020.559791. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 33015057 Free PMC article. Review.
-
A rare natural lipid induces neuroglobin expression to prevent amyloid oligomers toxicity and retinal neurodegeneration.Aging Cell. 2022 Jul;21(7):e13645. doi: 10.1111/acel.13645. Epub 2022 Jun 3. Aging Cell. 2022. PMID: 35656861 Free PMC article.
-
Conformational distortion in a fibril-forming oligomer arrests alpha-Synuclein fibrillation and minimizes its toxic effects.Commun Biol. 2021 May 3;4(1):518. doi: 10.1038/s42003-021-02026-z. Commun Biol. 2021. PMID: 33941845 Free PMC article.
-
Cellular models of alpha-synuclein toxicity and aggregation.J Neurochem. 2019 Sep;150(5):566-576. doi: 10.1111/jnc.14806. Epub 2019 Jul 30. J Neurochem. 2019. PMID: 31265132 Free PMC article. Review.
References
-
- de Rijk MC, Launer LJ, Berger K, Breteler MM, Dartigues JF, Baldereschi M, et al. Prevalence of Parkinson's disease in Europe: A collaborative study of population-based cohorts. Neurologic Diseases in the Elderly Research Group. Neurology. 2000;54(11 Suppl 5):S21–3. - PubMed
-
- German DC, Manaye K, Smith WK, Woodward DJ, Saper CB. Midbrain dopaminergic cell loss in Parkinson's disease: computer visualization. Ann Neurol. 1989;26(4):507–14. - PubMed
-
- German DC, Manaye KF, Sonsalla PK, Brooks BA. Midbrain dopaminergic cell loss in Parkinson's disease and MPTP-induced parkinsonism: sparing of calbindin-D28k-containing cells. Ann N Y Acad Sci. 1992;648:42–62. - PubMed
-
- Fedorow H, Tribl F, Halliday G, Gerlach M, Riederer P, Double KL. Neuromelanin in human dopamine neurons: comparison with peripheral melanins and relevance to Parkinson's disease. Progress in neurobiology. 2005;75(2):109–24. Epub 2005/03/24. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

