Identification of Senescent Cells in the Bone Microenvironment
- PMID: 27341653
- PMCID: PMC5289710
- DOI: 10.1002/jbmr.2892
Identification of Senescent Cells in the Bone Microenvironment
Abstract
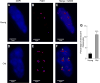
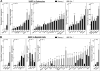
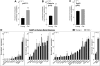
Cellular senescence is a fundamental mechanism by which cells remain metabolically active yet cease dividing and undergo distinct phenotypic alterations, including upregulation of p16Ink4a , profound secretome changes, telomere shortening, and decondensation of pericentromeric satellite DNA. Because senescent cells accumulate in multiple tissues with aging, these cells and the dysfunctional factors they secrete, termed the senescence-associated secretory phenotype (SASP), are increasingly recognized as promising therapeutic targets to prevent age-related degenerative pathologies, including osteoporosis. However, the cell type(s) within the bone microenvironment that undergoes senescence with aging in vivo has remained poorly understood, largely because previous studies have focused on senescence in cultured cells. Thus in young (age 6 months) and old (age 24 months) mice, we measured senescence and SASP markers in vivo in highly enriched cell populations, all rapidly isolated from bone/marrow without in vitro culture. In both females and males, p16Ink4a expression by real-time quantitative polymerase chain reaction (rt-qPCR) was significantly higher with aging in B cells, T cells, myeloid cells, osteoblast progenitors, osteoblasts, and osteocytes. Further, in vivo quantification of senescence-associated distension of satellites (SADS), ie, large-scale unraveling of pericentromeric satellite DNA, revealed significantly more senescent osteocytes in old compared with young bone cortices (11% versus 2%, p < 0.001). In addition, primary osteocytes from old mice had sixfold more (p < 0.001) telomere dysfunction-induced foci (TIFs) than osteocytes from young mice. Corresponding with the age-associated accumulation of senescent osteocytes was significantly higher expression of multiple SASP markers in osteocytes from old versus young mice, several of which also showed dramatic age-associated upregulation in myeloid cells. These data show that with aging, a subset of cells of various lineages within the bone microenvironment become senescent, although senescent myeloid cells and senescent osteocytes predominantly develop the SASP. Given the critical roles of osteocytes in orchestrating bone remodeling, our findings suggest that senescent osteocytes and their SASP may contribute to age-related bone loss. © 2016 American Society for Bone and Mineral Research.
Keywords: AGING; ANIMAL MODELS; CELL/TISSUE SIGNALING; OSTEOCYTES.
© 2016 American Society for Bone and Mineral Research.
Conflict of interest statement
All authors state that they have no conflicts of interest.
Figures
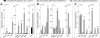


Comment in
-
Senescent Osteocytes: Do They Cause Damage and Can They Be Targeted to Preserve the Skeleton?J Bone Miner Res. 2016 Nov;31(11):1917-1919. doi: 10.1002/jbmr.2994. Epub 2016 Oct 6. J Bone Miner Res. 2016. PMID: 27653182 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

