Antiviral activity of Lactobacillus reuteri Protectis against Coxsackievirus A and Enterovirus 71 infection in human skeletal muscle and colon cell lines
- PMID: 27341804
- PMCID: PMC4920999
- DOI: 10.1186/s12985-016-0567-6
Antiviral activity of Lactobacillus reuteri Protectis against Coxsackievirus A and Enterovirus 71 infection in human skeletal muscle and colon cell lines
Erratum in
-
Erratum to: Antiviral activity of Lactobacillus reuteri Protectis against Coxsackievirus A and Enterovirus 71 infection in human skeletal muscle and colon cell lines.Virol J. 2016 Nov 17;13(1):186. doi: 10.1186/s12985-016-0633-0. Virol J. 2016. PMID: 27855715 Free PMC article. No abstract available.
Abstract
Background: Recurrence of hand, foot and mouth disease (HFMD) pandemics continues to threaten public health. Despite increasing awareness and efforts, effective vaccine and drug treatment have yet to be available. Probiotics have gained recognition in the field of healthcare worldwide, and have been extensively prescribed to babies and young children to relieve gastrointestinal (GI) disturbances and diseases, associated or not with microbial infections. Since the faecal-oral axis represents the major route of HFMD transmission, transient persistence of probiotic bacteria in the GI tract may confer some protection against HFMD and limit transmission among children.
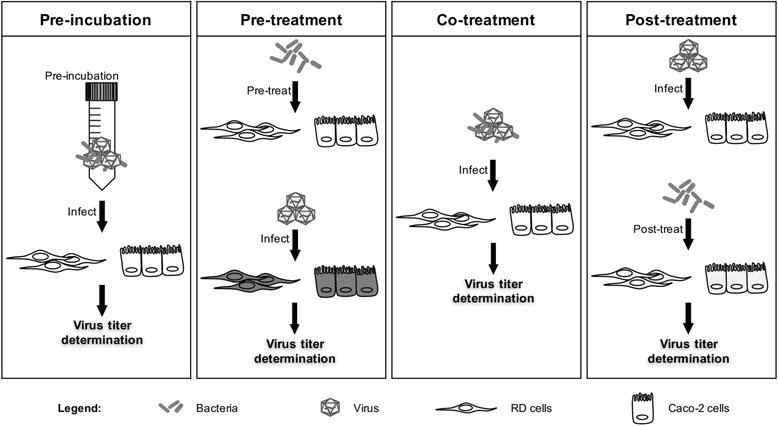
Methods: In this work, the antiviral activity of two commercially available probiotics, namely Lactobacillus reuteri Protectis (L. reuteri Protectis) and Lactobacillus casei Shirota (L. casei Shirota), was assayed against Coxsackieviruses and Enterovirus 71 (EV71), the main agents responsible for HFMD. In vitro infection set-ups using human skeletal muscle and colon cell lines were designed to assess the antiviral effect of the probiotic bacteria during entry and post-entry steps of the infection cycle.
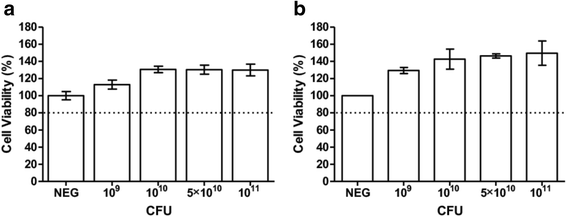
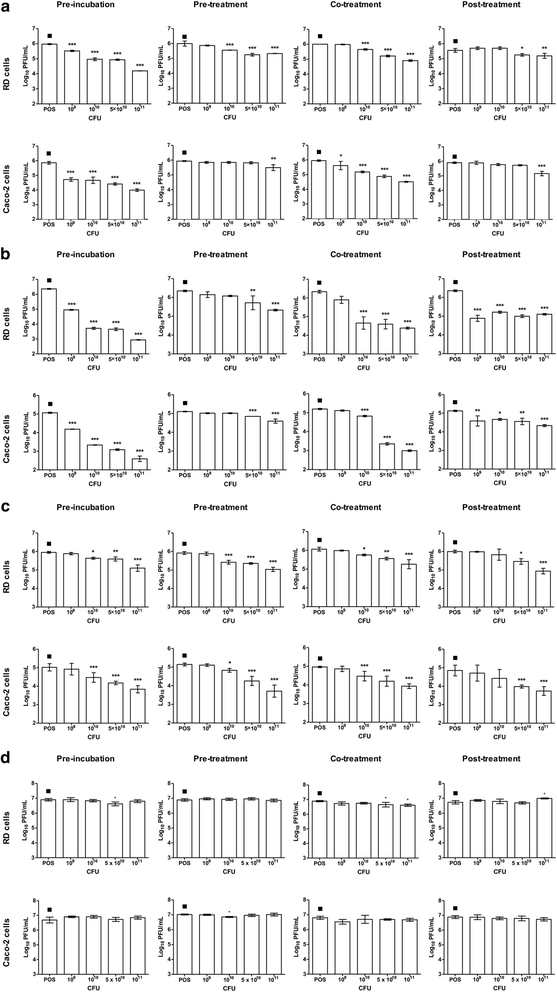
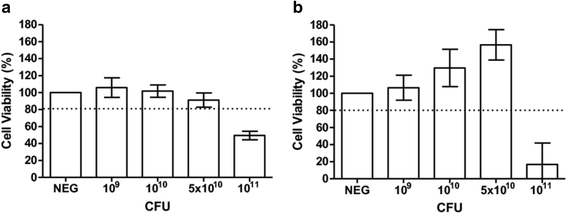
Results: Our findings indicate that L. reuteri Protectis displays a significant dose-dependent antiviral activity against Coxsackievirus type A (CA) strain 6 (CA6), CA16 and EV71, but not against Coxsackievirus type B strain 2. Our data support that the antiviral effect is likely achieved through direct physical interaction between bacteria and virus particles, which impairs virus entry into its mammalian host cell. In contrast, no significant antiviral effect was observed with L. casei Shirota.
Conclusions: Should the antiviral activity of L. reuteri Protectis observed in vitro be translated in vivo, such probiotics-based therapeutic approach may have the potential to address the urgent need for a safe and effective means to protect against HFMD and limit its transmission among children.
Keywords: Coxsackievirus; Enterovirus 71; Foot and mouth disease; Hand; Lactobacillus reuteri; Probiotics.
Figures




References
-
- Bruu AL: Enteroviruses: Polioviruses, coxsackieviruses, echoviruses, and newer enteroviruses. A Practical Guide to Clinical Virology, Second Edition 2003;44-45. doi:10.1002/0470857285.ch6
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

