Loss of Junctional Adhesion Molecule A Promotes Severe Steatohepatitis in Mice on a Diet High in Saturated Fat, Fructose, and Cholesterol
- PMID: 27342212
- PMCID: PMC5037035
- DOI: 10.1053/j.gastro.2016.06.022
Loss of Junctional Adhesion Molecule A Promotes Severe Steatohepatitis in Mice on a Diet High in Saturated Fat, Fructose, and Cholesterol
Abstract
Background & aims: There is evidence from clinical studies that compromised intestinal epithelial permeability contributes to the development of nonalcoholic steatohepatitis (NASH), but the exact mechanisms are not clear. Mice with disruption of the gene (F11r) encoding junctional adhesion molecule A (JAM-A) have defects in intestinal epithelial permeability. We used these mice to study how disruption of the intestinal epithelial barrier contributes to NASH.
Methods: Male C57BL/6 (control) or F11r(-/-) mice were fed a normal diet or a diet high in saturated fat, fructose, and cholesterol (HFCD) for 8 weeks. Liver and intestinal tissues were collected and analyzed by histology, quantitative reverse-transcription polymerase chain reaction, and flow cytometry. Intestinal epithelial permeability was assessed in mice by measuring permeability to fluorescently labeled dextran. The intestinal microbiota were analyzed using 16S ribosomal RNA sequencing. We also analyzed biopsy specimens from proximal colons of 30 patients with nonalcoholic fatty liver disease (NAFLD) and 19 subjects without NAFLD (controls) undergoing surveillance colonoscopy.
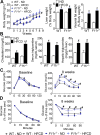
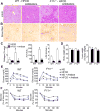
Results: F11r(-/-) mice fed a HFCD, but not a normal diet, developed histologic and pathologic features of severe NASH including steatosis, lobular inflammation, hepatocellular ballooning, and fibrosis, whereas control mice fed a HFCD developed only modest steatosis. Interestingly, there were no differences in body weight, ratio of liver weight:body weight, or glucose homeostasis between control and F11r(-/-) mice fed a HFCD. In these mice, liver injury was associated with significant increases in mucosal inflammation, tight junction disruption, and intestinal epithelial permeability to bacterial endotoxins, compared with control mice or F11r(-/-) mice fed a normal diet. The HFCD led to a significant increase in inflammatory microbial taxa in F11r(-/-) mice, compared with control mice. Administration of oral antibiotics or sequestration of bacterial endotoxins with sevelamer hydrochloride reduced mucosal inflammation and restored normal liver histology in F11r(-/-) mice fed a HFCD. Protein and transcript levels of JAM-A were significantly lower in the intestinal mucosa of patients with NAFLD than without NAFLD; decreased expression of JAM-A correlated with increased mucosal inflammation.
Conclusions: Mice with defects in intestinal epithelial permeability develop more severe steatohepatitis after a HFCD than control mice, and colon tissues from patients with NAFLD have lower levels of JAM-A and higher levels of inflammation than subjects without NAFLD. These findings indicate that intestinal epithelial barrier function and microbial dysbiosis contribute to the development of NASH. Restoration of intestinal barrier integrity and manipulation of gut microbiota might be developed as therapeutic strategies for patients with NASH.
Keywords: Bacterial Translocation; Claudin-4; Occludin.
Copyright © 2016 AGA Institute. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
All authors declare no conflicting interests.
Figures




References
-
- Mahady SE, George J. Management of nonalcoholic steatohepatitis: an evidence-based approach. Clin Liver Dis. 2012;16:631–45. - PubMed
-
- Williams CD, Stengel J, Asike MI, et al. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: a prospective study. Gastroenterology. 2011;140:124–31. - PubMed
-
- Miele L, Valenza V, La Torre G, et al. Increased intestinal permeability and tight junction alterations in nonalcoholic fatty liver disease. Hepatology. 2009;49:1877–87. - PubMed
-
- Lorenzo-Zuniga V, Bartoli R, Planas R, et al. Oral bile acids reduce bacterial overgrowth, bacterial translocation, and endotoxemia in cirrhotic rats. Hepatology. 2003;37:551–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

