Preanalytical conditions of point-of-care testing in the intensive care unit are decisive for analysis reliability
- PMID: 27342259
- PMCID: PMC4920790
- DOI: 10.1186/s13613-016-0152-6
Preanalytical conditions of point-of-care testing in the intensive care unit are decisive for analysis reliability
Abstract
Background: Point-of-care testing (POCT) systems enable a wide range of tests to be rapidly performed at the bedside and have attracted increasing interest in the intensive care unit (ICU). However, previous studies comparing the concordance of POCT with central laboratory testing have reported divergent findings. Most reported studies on POCT reliability have focused on analyzer performance rather than the preanalytical phase. The aim of this study was to assess the reliability of results provided by point-of-care analyzers according to the organization of the care units and the preanalytical process.
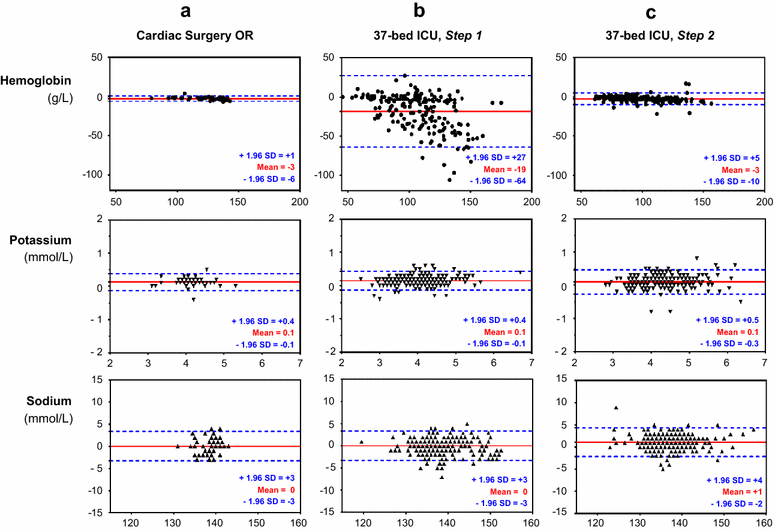
Methods: In three adult critical care units, 491 paired blood samples were analyzed for hemoglobin, potassium, and sodium concentrations by blood gas analyzers (identical reference) and the central laboratory. The clinical significance of agreement was assessed using Bland-Altman plots. A quality improvement program was then implemented to improve the preanalytical POCT process for one ICU where there was poor agreement. A second comparison was performed on 278 paired blood samples in this unit.
Results: Biases were clinically nonsignificant for potassium and sodium concentrations for all tested critical care units, relative to the reference method. However, biases [limits of agreements] for hemoglobin analyses were clearly affected by the preanalytical process: -3 [-6; 1] g/L in the operating room, -5 [-28; 17] g/L in a 10-bed ICU, and -19 [-64; 27] g/L in a 37-bed ICU. The quality approach was implemented in the 37-bed ICU and led to corrective actions that: (1) reduced the time for the POCT preanalytical phase; (2) implemented a checklist to validate the preanalytical conditions; (3) used technical innovations. The improvement of the preanalytical process resulted in a substantial decrease of the bias for hemoglobin concentration measurements: -3 [-10; 5] g/L in the 37-bed ICU.
Conclusion: We clearly demonstrate that an identical analyzer can provide results of varying quality depending on the local constraints of the ICUs. We demonstrate that quality management focused on the preanalytical process and performed by the partners involved in the POCT can overcome these issues.
Keywords: Electrolyte; Hemoglobin; Intensive care; Point-of-care testing; Preanalytical.
Figures
References
-
- Schwarzer P, Kuhn S-O, Stracke S, Gründling M, Knigge S, Selleng S, Helm M, Friesecke S, Abel P, Kallner A, Nauck M, Petersmann A. Discrepant post filter ionized calcium concentrations by common blood gas analyzers in CRRT using regional citrate anticoagulation. Crit Care. 2015;19:321. doi: 10.1186/s13054-015-1027-1. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

