Idelalisib Impacts Cell Growth through Inhibiting Translation-Regulatory Mechanisms in Mantle Cell Lymphoma
- PMID: 27342398
- PMCID: PMC5433851
- DOI: 10.1158/1078-0432.CCR-15-3135
Idelalisib Impacts Cell Growth through Inhibiting Translation-Regulatory Mechanisms in Mantle Cell Lymphoma
Abstract
Purpose: PI3K is a critical node in the B-cell receptor pathway, which is responsible for survival and proliferation of B-cell malignancies. Idelalisib, a PI3Kδ-isoform-specific inhibitor, has been approved to treat B-cell malignancies. Although biological activity of the drug has been evaluated, molecular mechanisms and signaling pathway disruption leading to the biological effects of idelalisib are not yet well defined. Prior laboratory reports have identified transcription and translation as the primary events for attenuation of PI3Kα isoform. We hypothesized that PI3Kδ-isoform inhibition by idelalisib should also affect gene transcription and protein translation.
Experimental design: Using three mantle cell lymphoma cell lines and primary cells from patients, biological consequences such as apoptosis/cell-cycle analysis, as well as RNA/protein synthesis were evaluated. Proteomics analyses (RPPA and immunoblot assays) defined molecular events downstream of PI3K/AKT cassette.
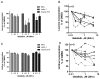
Results: Idelalisib treatment resulted in inhibition of protein synthesis, which correlated with reduction in cell size and cell growth. A moderate loss of viability without any change in cell-cycle profile was observed. Idelalisib treatment inhibited AKT activation, an immediate downstream PI3K effector, and also reduced phosphorylation levels of downstream AKT/mTOR pathway proteins such as PRAS40. In addition, idelalisib treatment impeded activation of the MAPK pathway, and MEK, ERK and p90RSK phosphorylation levels were reduced. Reduction in AKT, PDK1, and MEK phosphorylation correlated with protein synthesis inhibition.
Conclusions: Collectively, these results clarify the molecular mechanisms of actions and may provide biomarkers and targets for combination with idelalisib in B-cell malignancies. Clin Cancer Res; 23(1); 181-92. ©2016 AACR.
©2016 American Association for Cancer Research.
Conflict of interest statement
VG had a sponsored research agreement with Gilead Sciences. The other authors do not have any conflict of interest.
Figures
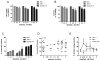







References
-
- Okkenhaug K, Bilancio A, Farjot G, Priddle H, Sancho S, Peskett E, et al. Impaired B and T cell antigen receptor signaling in p110delta PI 3-kinase mutant mice. Science. 2002;297:1031–4. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

