Iron-related parameters in dialysis patients treated with sucroferric oxyhydroxide
- PMID: 27342579
- PMCID: PMC5837623
- DOI: 10.1093/ndt/gfw242
Iron-related parameters in dialysis patients treated with sucroferric oxyhydroxide
Abstract
Background: Sucroferric oxyhydroxide is a non-calcium, iron-based phosphate binder indicated for the treatment of hyperphosphataemia in adult dialysis patients. This post hoc analysis of a randomized, 24-week Phase 3 study and its 28-week extension was performed to evaluate the long-term effect of sucroferric oxyhydroxide on iron parameters.
Methods: A total of 1059 patients were randomized to sucroferric oxyhydroxide 1.0-3.0 g/day (n = 710) or sevelamer carbonate ('sevelamer') 2.4-14.4 g/day (n = 349) for up to 52 weeks. The current analysis only included patients who completed 52 weeks of continuous treatment (n = 549). Changes in iron-related parameters and anti-anaemic product use during the study were measured.
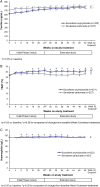
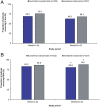
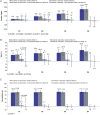
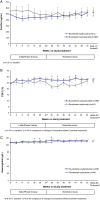
Results: Some changes in iron-related parameters across both treatment groups were observed during the first 24 weeks of the study, and to a lesser extent with longer-term treatment. There were small, but significantly greater increases in mean transferrin saturation (TSAT) and haemoglobin levels with sucroferric oxyhydroxide versus sevelamer during the first 24 weeks (change in TSAT: +4.6% versus +0.6%, P = 0.003; change in haemoglobin: +1.6 g/L versus -1.1 g/L, P = 0.037). Mean serum ferritin concentrations also increased from Weeks 0 to 24 with sucroferric oxyhydroxide and sevelamer (+119 ng/mL and +56.2 ng/mL respectively; no statistically significant difference between groups). In both treatment groups, ferritin concentrations increased to a greater extent in the overall study population [>70% of whom received concomitant intravenous (IV) iron], compared with the subset of patients who did not receive IV iron therapy during the study. The pattern of anti-anaemic product use was similar in both treatment groups, with a trend towards higher use of IV iron and erythropoiesis-stimulating agents with sevelamer.
Conclusions: Initial increases in some iron-related parameters were observed in both treatment groups but were more pronounced with sucroferric oxyhydroxide. These differences between treatment groups with respect to changes in iron parameters are likely due to minimal iron absorption from sucroferric oxyhydroxide.
Keywords: chronic kidney disease; dialysis; iron; phosphate binder; sucroferric oxyhydroxide.
© The Author 2016. Published by Oxford University Press on behalf of ERA-EDTA.
Figures
References
-
- Sprague SM, Covic A, Floege J. et al. Concomitant intravenous iron use drives changes in iron indices in a phase 3 study of PA21 Presented at: National Kidney Foundation, Las Vegas, NV, USA, 22‒26 April 2014
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

