Theranostic near-infrared fluorescent nanoplatform for imaging and systemic siRNA delivery to metastatic anaplastic thyroid cancer
- PMID: 27342857
- PMCID: PMC4948349
- DOI: 10.1073/pnas.1605841113
Theranostic near-infrared fluorescent nanoplatform for imaging and systemic siRNA delivery to metastatic anaplastic thyroid cancer
Abstract
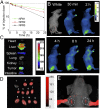
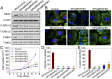
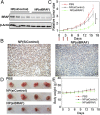
Anaplastic thyroid cancer (ATC), one of the most aggressive solid tumors, is characterized by rapid tumor growth and severe metastasis to other organs. Owing to the lack of effective treatment options, ATC has a mortality rate of ∼100% and median survival of less than 5 months. RNAi nanotechnology represents a promising strategy for cancer therapy through nanoparticle (NP) -mediated delivery of RNAi agents (e.g., siRNA) to solid tumors for specific silencing of target genes driving growth and/or metastasis. Nevertheless, the clinical success of RNAi cancer nanotherapies remains elusive in large part because of the suboptimal systemic siRNA NP delivery to tumors and the fact that tumor heterogeneity produces variable NP accumulation and thus, therapeutic response. To address these challenges, we here present an innovative theranostic NP platform composed of a near-infrared (NIR) fluorescent polymer for effective in vivo siRNA delivery to ATC tumors and simultaneous tracking of the tumor accumulation by noninvasive NIR imaging. The NIR polymeric NPs are small (∼50 nm), show long blood circulation and high tumor accumulation, and facilitate tumor imaging. Systemic siRNA delivery using these NPs efficiently silences the expression of V-Raf murine sarcoma viral oncogene homolog B (BRAF) in tumor tissues and significantly suppresses tumor growth and metastasis in an orthotopic mouse model of ATC. These results suggest that this theranostic NP system could become an effective tool for NIR imaging-guided siRNA delivery for personalized treatment of advanced malignancies.
Keywords: NIR imaging; anaplastic thyroid cancer; nanoparticle; siRNA delivery; theranostic.
Conflict of interest statement
Conflict of interest statement: O.C.F. discloses financial interest in BIND Therapeutics, Selecta Biosciences, and Tarveda Therapeutics.
Figures


Similar articles
-
Optical diagnostic imaging and therapy for thyroid cancer.Mater Today Bio. 2022 Sep 26;17:100441. doi: 10.1016/j.mtbio.2022.100441. eCollection 2022 Dec 15. Mater Today Bio. 2022. PMID: 36388462 Free PMC article. Review.
-
Single agent nanoparticle for radiotherapy and radio-photothermal therapy in anaplastic thyroid cancer.Biomaterials. 2015 Jul;57:41-9. doi: 10.1016/j.biomaterials.2015.04.013. Epub 2015 Apr 24. Biomaterials. 2015. PMID: 25913249 Free PMC article.
-
A sequential targeting nanoplatform for anaplastic thyroid carcinoma theranostics.Acta Biomater. 2020 Jan 15;102:367-383. doi: 10.1016/j.actbio.2019.11.043. Epub 2019 Nov 26. Acta Biomater. 2020. PMID: 31778831
-
Theranostic poly(lactic-co-glycolic acid) nanoparticle for magnetic resonance/infrared fluorescence bimodal imaging and efficient siRNA delivery to macrophages and its evaluation in a kidney injury model.Nanomedicine. 2017 Nov;13(8):2451-2462. doi: 10.1016/j.nano.2017.08.007. Epub 2017 Aug 24. Nanomedicine. 2017. PMID: 28842376
-
Personalized therapy in patients with anaplastic thyroid cancer: targeting genetic and epigenetic alterations.J Clin Endocrinol Metab. 2015 Jan;100(1):35-42. doi: 10.1210/jc.2014-2803. J Clin Endocrinol Metab. 2015. PMID: 25347569 Free PMC article. Review.
Cited by
-
Nanoparticles for siRNA-Based Gene Silencing in Tumor Therapy.IEEE Trans Nanobioscience. 2016 Dec;15(8):849-863. doi: 10.1109/TNB.2016.2621730. IEEE Trans Nanobioscience. 2016. PMID: 28092499 Free PMC article. Review.
-
Advances in Biomarker-Driven Targeted Therapies in Thyroid Cancer.Cancers (Basel). 2021 Dec 9;13(24):6194. doi: 10.3390/cancers13246194. Cancers (Basel). 2021. PMID: 34944814 Free PMC article. Review.
-
Metastatic disease in head & neck oncology.Acta Otorhinolaryngol Ital. 2020 Apr;40(SUPPL. 1):S1-S86. doi: 10.14639/0392-100X-suppl.1-40-2020. Acta Otorhinolaryngol Ital. 2020. PMID: 32469009 Free PMC article. Review.
-
Engineering Multifunctional RNAi Nanomedicine To Concurrently Target Cancer Hallmarks for Combinatorial Therapy.Angew Chem Int Ed Engl. 2018 Feb 5;57(6):1510-1513. doi: 10.1002/anie.201710144. Epub 2018 Jan 16. Angew Chem Int Ed Engl. 2018. PMID: 29276823 Free PMC article.
-
Optical diagnostic imaging and therapy for thyroid cancer.Mater Today Bio. 2022 Sep 26;17:100441. doi: 10.1016/j.mtbio.2022.100441. eCollection 2022 Dec 15. Mater Today Bio. 2022. PMID: 36388462 Free PMC article. Review.
References
-
- Smallridge RC, et al. American Thyroid Association Anaplastic Thyroid Cancer Guidelines Taskforce American Thyroid Association guidelines for management of patients with anaplastic thyroid cancer. Thyroid. 2012;22(11):1104–1139. - PubMed
-
- Kebebew E, Greenspan FS, Clark OH, Woeber KA, McMillan A. Anaplastic thyroid carcinoma. Treatment outcome and prognostic factors. Cancer. 2005;103(7):1330–1335. - PubMed
-
- Zuckerman JE, Davis ME. Clinical experiences with systemically administered siRNA-based therapeutics in cancer. Nat Rev Drug Discov. 2015;14(12):843–856. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

