ATP: The crucial component of secretory vesicles
- PMID: 27342860
- PMCID: PMC4948319
- DOI: 10.1073/pnas.1600690113
ATP: The crucial component of secretory vesicles
Abstract
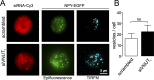
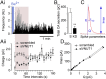
The colligative properties of ATP and catecholamines demonstrated in vitro are thought to be responsible for the extraordinary accumulation of solutes inside chromaffin cell secretory vesicles, although this has yet to be demonstrated in living cells. Because functional cells cannot be deprived of ATP, we have knocked down the expression of the vesicular nucleotide carrier, the VNUT, to show that a reduction in vesicular ATP is accompanied by a drastic fall in the quantal release of catecholamines. This phenomenon is particularly evident in newly synthesized vesicles, which we show are the first to be released. Surprisingly, we find that inhibiting VNUT expression also reduces the frequency of exocytosis, whereas the overexpression of VNUT drastically increases the quantal size of exocytotic events. To our knowledge, our data provide the first demonstration that ATP, in addition to serving as an energy source and purinergic transmitter, is an essential element in the concentration of catecholamines in secretory vesicles. In this way, cells can use ATP to accumulate neurotransmitters and other secreted substances at high concentrations, supporting quantal transmission.
Keywords: VNUT; exocytosis; purines; quantum size; secretory vesicles.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
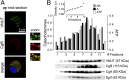
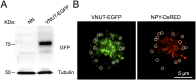


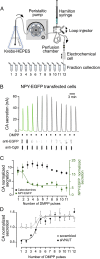

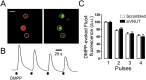
Comment in
-
Commentary: ATP: The crucial component of secretory vesicles: Accelerated ATP/insulin exocytosis and prediabetes.Front Physiol. 2017 Feb 2;8:53. doi: 10.3389/fphys.2017.00053. eCollection 2017. Front Physiol. 2017. PMID: 28210227 Free PMC article. No abstract available.
References
-
- Burnstock G. Historical review: ATP as a neurotransmitter. Trends Pharmacol Sci. 2006;27(3):166–176. - PubMed
-
- Johnson RG, Carlson NJ, Scarpa A. deltapH and catecholamine distribution in isolated chromaffin granules. J Biol Chem. 1978;253(5):1512–1521. - PubMed
-
- Albillos A, et al. The exocytotic event in chromaffin cells revealed by patch amperometry. Nature. 1997;389(6650):509–512. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

