Dose dependency of outcomes of intrapleural fibrinolytic therapy in new rabbit empyema models
- PMID: 27343192
- PMCID: PMC5142452
- DOI: 10.1152/ajplung.00171.2016
Dose dependency of outcomes of intrapleural fibrinolytic therapy in new rabbit empyema models
Abstract
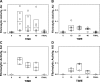
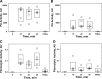
The incidence of empyema (EMP) is increasing worldwide; EMP generally occurs with pleural loculation and impaired drainage is often treated with intrapleural fibrinolytic therapy (IPFT) or surgery. A number of IPFT options are used clinically with empiric dosing and variable outcomes in adults. To evaluate mechanisms governing intrapleural fibrinolysis and disease outcomes, models of Pasteurella multocida and Streptococcus pneumoniae were generated in rabbits and the animals were treated with either human tissue (tPA) plasminogen activator or prourokinase (scuPA). Rabbit EMP was characterized by the development of pleural adhesions detectable by chest ultrasonography and fibrinous coating of the pleura. Similar to human EMP, rabbits with EMP accumulated sizable, 20- to 40-ml fibrinopurulent pleural effusions associated with extensive intrapleural organization, significantly increased pleural thickness, suppression of fibrinolytic and plasminogen-activating activities, and accumulation of high levels of plasminogen activator inhibitor 1, plasminogen, and extracellular DNA. IPFT with tPA (0.145 mg/kg) or scuPA (0.5 mg/kg) was ineffective in rabbit EMP (n = 9 and 3 for P. multocida and S. pneumoniae, respectively); 2 mg/kg tPA or scuPA IPFT (n = 5) effectively cleared S. pneumoniae-induced EMP collections in 24 h with no bleeding observed. Although intrapleural fibrinolytic activity for up to 40 min after IPFT was similar for effective and ineffective doses of fibrinolysin, it was lower for tPA than for scuPA treatments. These results demonstrate similarities between rabbit and human EMP, the importance of pleural fluid PAI-1 activity, and levels of plasminogen in the regulation of intrapleural fibrinolysis and illustrate the dose dependency of IPFT outcomes in EMP.
Keywords: empyema; fibrinolysis; plasminogen activator inhibitor-1; single chain urokinase; tissue plasminogen activator.
Copyright © 2016 the American Physiological Society.
Figures









References
-
- Abu-Daff S, Maziak DE, Alshehab D, Threader J, Ivanovic J, Deslaurier V, Villeneuve PJ, Gilbert S, Sundaresan S, Shamji F, Lougheed C, Seely JM, Seely AJ. Intrapleural fibrinolytic therapy (IPFT) in loculated pleural effusions—analysis of predictors for failure of therapy and bleeding: a cohort study. BMJ Open 3: 2013. - PMC - PubMed
-
- Asselbergs FW, Williams SM, Hebert PR, Coffey CS, Hillege HL, Navis G, Vaughan DE, van Gilst WH, Moore JH. The gender-specific role of polymorphisms from the fibrinolytic, renin-angiotensin, and bradykinin systems in determining plasma t-PA and PAI-1 levels. Thromb Haemost 96: 471–477, 2006. - PubMed
-
- Bouros D, Antoniou KM, Chalkiadakis G, Drositis J, Petrakis I, Siafakas N. The role of video-assisted thoracoscopic surgery in the treatment of parapneumonic empyema after the failure of fibrinolytics. Surg Endosc 16: 151–154, 2002. - PubMed
-
- Chiu CY, Wong KS, Huang JL, Tasi MH, Lin TY, Hsieh SY. Proinflammatory cytokines, fibrinolytic system enzymes, and biochemical indices in children with infectious para-pneumonic effusions. Pediatr Infect Dis J 27: 699–703, 2008. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

