MiR-196a regulates heme oxygenase-1 by silencing Bach1 in the neonatal mouse lung
- PMID: 27343195
- PMCID: PMC5142454
- DOI: 10.1152/ajplung.00428.2015
MiR-196a regulates heme oxygenase-1 by silencing Bach1 in the neonatal mouse lung
Abstract
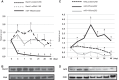
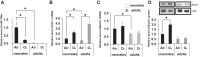
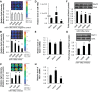
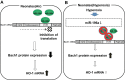
In the lung, heme oxygenase-1 (HO-1) is developmentally regulated, with its highest expression in the first days of life. In addition, neonatal mice have limited HO-1 induction in hyperoxia compared with adults. However, few reports have addressed the functional effect of microRNAs (miRNAs) in the regulation of HO-1 in vivo. The aims of the present study were to characterize changes in lung miRNA expression during postnatal development and in response to hyperoxic exposure, and to identify miRNAs that target lung HO-1 gene expression. Neonatal (<12 h old) and adult (2 mo old) mice were exposed to room air or hyperoxia (95% oxygen) for 72 h. TaqMan low-density array rodent miRNA assays were used to calculate miRNA expression changes between control and hyperoxia groups in neonatal and adult lungs. In neonates, we identified miR-196a, which binds to the 3'-untranslated region of the transcriptional repressor BTB and CNC homology 1 (Bach1) and regulates its expression, and subsequently leads to higher levels of lung HO-1 mRNA compared with levels in adults. Despite the increase at baseline, miR-196a was degraded in hyperoxia resulting in limited HO-1 induction in neonatal mice lungs. Furthermore, the developmental differences in lung HO-1 gene expression can be explained in part by the variation in miRNA-196a and its effect on Bach1. This report is the first to show developmental differences in lung miR-196a and its effect on Bach1 and HO-1 expression at baseline and in hyperoxia.
Keywords: Bach1; HO-1; hyperoxic lung injury; lung development; microRNA-196a.
Copyright © 2016 the American Physiological Society.
Figures





Similar articles
-
Bach1 modulates heme oxygenase-1 expression in the neonatal mouse lung.Pediatr Res. 2009 Feb;65(2):145-9. doi: 10.1203/PDR.0b013e318191eedc. Pediatr Res. 2009. PMID: 18948842 Free PMC article.
-
Genetic ablation of Bach1 gene enhances recovery from hyperoxic lung injury in newborn mice via transient upregulation of inflammatory genes.Pediatr Res. 2017 Jun;81(6):926-931. doi: 10.1038/pr.2017.17. Epub 2017 Jan 18. Pediatr Res. 2017. PMID: 28099425
-
Increased expression of heme oxygenase-1 suppresses airway branching morphogenesis in fetal mouse lungs exposed to inflammation.Pediatr Res. 2020 Feb;87(3):494-500. doi: 10.1038/s41390-019-0588-0. Epub 2019 Oct 2. Pediatr Res. 2020. PMID: 31578032
-
Heme oxygenase in neonatal lung injury and repair.Antioxid Redox Signal. 2014 Nov 1;21(13):1881-92. doi: 10.1089/ars.2013.5791. Epub 2014 Feb 19. Antioxid Redox Signal. 2014. PMID: 24382006 Free PMC article. Review.
-
BACH1, the master regulator gene: A novel candidate target for cancer therapy.Gene. 2016 Aug 15;588(1):30-7. doi: 10.1016/j.gene.2016.04.040. Epub 2016 Apr 22. Gene. 2016. PMID: 27108804 Review.
Cited by
-
Heme Oxygenase 1 as a Therapeutic Target in Acute Kidney Injury.Am J Kidney Dis. 2017 Apr;69(4):531-545. doi: 10.1053/j.ajkd.2016.10.037. Epub 2017 Jan 27. Am J Kidney Dis. 2017. PMID: 28139396 Free PMC article. Review.
-
Cell death or survival: Insights into the role of mRNA translational control.Semin Cell Dev Biol. 2024 Feb 15;154(Pt B):138-154. doi: 10.1016/j.semcdb.2023.06.006. Epub 2023 Jun 23. Semin Cell Dev Biol. 2024. PMID: 37357122 Free PMC article. Review.
-
Alterations in sperm RNAs persist after alcohol cessation and correlate with epididymal mitochondrial dysfunction.Andrology. 2024 Jul;12(5):1012-1023. doi: 10.1111/andr.13566. Epub 2023 Dec 3. Andrology. 2024. PMID: 38044754 Free PMC article.
-
MicroRNAs and Synaptic Plasticity: From Their Molecular Roles to Response to Therapy.Mol Neurobiol. 2022 Aug;59(8):5084-5102. doi: 10.1007/s12035-022-02907-2. Epub 2022 Jun 6. Mol Neurobiol. 2022. PMID: 35666404 Review.
-
miR-196a Enhances Neuronal Morphology through Suppressing RANBP10 to Provide Neuroprotection in Huntington's Disease.Theranostics. 2017 Jun 24;7(9):2452-2462. doi: 10.7150/thno.18813. eCollection 2017. Theranostics. 2017. PMID: 28744327 Free PMC article.
References
-
- Abate A, Yang G, Wong RJ, Schroder H, Stevenson DK, Dennery PA. Apigenin decreases hemin-mediated heme oxygenase-1 induction. Free Radic Biol Med 15, 711–718, 2005. - PubMed
-
- Baraldi E, Filippone M. Chronic lung disease after premature birth. N Engl J Med 357: 1946–1955, 2007. - PubMed
-
- Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, function. Cell 116: 281–297, 2004. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

