v-SNARE transmembrane domains function as catalysts for vesicle fusion
- PMID: 27343350
- PMCID: PMC4972536
- DOI: 10.7554/eLife.17571
v-SNARE transmembrane domains function as catalysts for vesicle fusion
Abstract
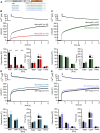
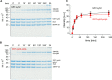
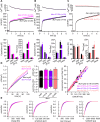
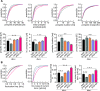
Vesicle fusion is mediated by an assembly of SNARE proteins between opposing membranes, but it is unknown whether transmembrane domains (TMDs) of SNARE proteins serve mechanistic functions that go beyond passive anchoring of the force-generating SNAREpin to the fusing membranes. Here, we show that conformational flexibility of synaptobrevin-2 TMD is essential for efficient Ca(2+)-triggered exocytosis and actively promotes membrane fusion as well as fusion pore expansion. Specifically, the introduction of helix-stabilizing leucine residues within the TMD region spanning the vesicle's outer leaflet strongly impairs exocytosis and decelerates fusion pore dilation. In contrast, increasing the number of helix-destabilizing, ß-branched valine or isoleucine residues within the TMD restores normal secretion but accelerates fusion pore expansion beyond the rate found for the wildtype protein. These observations provide evidence that the synaptobrevin-2 TMD catalyzes the fusion process by its structural flexibility, actively setting the pace of fusion pore expansion.
Keywords: exocytosis; membrane fusion; mouse; neuroscience; neurotransmitter release; synaptobrevin.
Conflict of interest statement
The authors declare that no competing interests exist.
Figures









Similar articles
-
A structural role for the synaptobrevin 2 transmembrane domain in dense-core vesicle fusion pores.J Neurosci. 2015 Apr 8;35(14):5772-80. doi: 10.1523/JNEUROSCI.3983-14.2015. J Neurosci. 2015. PMID: 25855187 Free PMC article.
-
The Transmembrane Domain of Synaptobrevin Influences Neurotransmitter Flux through Synaptic Fusion Pores.J Neurosci. 2018 Aug 8;38(32):7179-7191. doi: 10.1523/JNEUROSCI.0721-18.2018. Epub 2018 Jul 16. J Neurosci. 2018. PMID: 30012692 Free PMC article.
-
Synergistic actions of v-SNARE transmembrane domains and membrane-curvature modifying lipids in neurotransmitter release.Elife. 2020 May 11;9:e55152. doi: 10.7554/eLife.55152. Elife. 2020. PMID: 32391794 Free PMC article.
-
Fusion pore in exocytosis: More than an exit gate? A β-cell perspective.Cell Calcium. 2017 Dec;68:45-61. doi: 10.1016/j.ceca.2017.10.005. Epub 2017 Nov 7. Cell Calcium. 2017. PMID: 29129207 Review.
-
Role of PI(4,5)P(2) in vesicle exocytosis and membrane fusion.Subcell Biochem. 2012;59:111-30. doi: 10.1007/978-94-007-3015-1_4. Subcell Biochem. 2012. PMID: 22374089 Free PMC article. Review.
Cited by
-
Structural Roles for the Juxtamembrane Linker Region and Transmembrane Region of Synaptobrevin 2 in Membrane Fusion.Front Cell Dev Biol. 2021 Jan 6;8:609708. doi: 10.3389/fcell.2020.609708. eCollection 2020. Front Cell Dev Biol. 2021. PMID: 33490074 Free PMC article.
-
A Central Small Amino Acid in the VAMP2 Transmembrane Domain Regulates the Fusion Pore in Exocytosis.Sci Rep. 2017 Jun 6;7(1):2835. doi: 10.1038/s41598-017-03013-3. Sci Rep. 2017. PMID: 28588281 Free PMC article.
-
Toward a unified picture of the exocytotic fusion pore.FEBS Lett. 2018 Nov;592(21):3563-3585. doi: 10.1002/1873-3468.13270. Epub 2018 Oct 26. FEBS Lett. 2018. PMID: 30317539 Free PMC article. Review.
-
Synaptobrevin2 monomers and dimers differentially engage to regulate the functional trans-SNARE assembly.Life Sci Alliance. 2024 Jan 18;7(4):e202402568. doi: 10.26508/lsa.202402568. Print 2024 Apr. Life Sci Alliance. 2024. PMID: 38238088 Free PMC article.
-
Thermodynamically reversible paths of the first fusion intermediate reveal an important role for membrane anchors of fusion proteins.Proc Natl Acad Sci U S A. 2019 Feb 12;116(7):2571-2576. doi: 10.1073/pnas.1818200116. Epub 2019 Jan 30. Proc Natl Acad Sci U S A. 2019. PMID: 30700547 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

