Aripiprazole for autism spectrum disorders (ASD)
- PMID: 27344135
- PMCID: PMC7120220
- DOI: 10.1002/14651858.CD009043.pub3
Aripiprazole for autism spectrum disorders (ASD)
Abstract
Background: Autism spectrum disorders (ASD) include autistic disorder, Asperger's disorder and pervasive developmental disorder - not otherwise specified (PDD-NOS). Antipsychotics have been used as a medication intervention for irritability related to ASD. Aripiprazole, a third-generation, atypical antipsychotic, is a relatively new drug that has a unique mechanism of action different from that of other antipsychotics. This review updates a previous Cochrane review on the safety and efficacy of aripiprazole for individuals with ASD, published in 2011 (Ching 2011).
Objectives: To assess the safety and efficacy of aripiprazole as medication treatment for individuals with ASD.
Search methods: In October 2015, we searched the Cochrane Central Register of Controlled Trials (CENTRAL), Ovid MEDLINE, Embase, the Cumulative Index to Nursing and Allied Health Literature (CINAHL) and seven other databases as well as two trial registers. We searched for records published in 1990 or later, as this was the year aripiprazole became available.
Selection criteria: Randomised controlled trials (RCTs) of aripiprazole (administered orally and at any dosage) versus placebo for treatment of individuals with a diagnosis of ASD.
Data collection and analysis: Two review authors independently collected, evaluated and analysed data. We performed meta-analysis for primary and secondary outcomes, when possible. We used the GRADE (Grades of Recommendation, Assessment, Development and Evaluation) approach to rate the overall quality of the evidence.
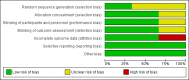
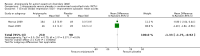
Main results: We included three trials in this review. Two were included in the previous published review, and the results of one, placebo-controlled discontinuation study were added to this review. Although we searched for studies across age groups, we found only studies conducted in children and youth. Included trials had low risk of bias across most domains. High risk of bias was seen in only one trial with incomplete outcome data. We judged the overall quality of the evidence for most outcomes to be moderate.Two RCTs with similar methods evaluated use of aripiprazole for a duration of eight weeks in 316 children/adolescents with ASD. Meta-analysis of study results revealed a mean improvement of -6.17 points on the Aberrant Behavior Checklist (ABC) - Irritability subscale (95% confidence intervals (CIs) -9.07 to -3.26, two studies, 308 children/adolescents, moderate-quality evidence), -7.93 points on the ABC - Hyperactivity subscale (95% CI -10.98 to -4.88, two studies, 308 children/adolescents, moderate-quality evidence) and -2.66 points on the ABC - Stereotypy subscale (95% CI -3.55 to -1.77, two studies, 308 children/adolescents, moderate-quality evidence) in children/adolescents taking aripiprazole relative to children/adolescents taking placebo. In terms of side effects, children/adolescents taking aripiprazole had a greater increase in weight, with a mean increase of 1.13 kg relative to placebo (95% CI 0.71 to 1.54, two studies, 308 children/adolescents, moderate-quality evidence), and had a higher risk ratio (RR) for sedation (RR 4.28, 95% CI 1.58 to 11.60, two studies, 313 children/adolescents, moderate-quality evidence) and tremor (RR 10.26, 95% CI 1.37 to 76.63, two studies, 313 children/adolescents, moderate-quality evidence). A randomised, placebo-controlled discontinuation study found that 35% of children/adolescents randomised to continue intervention with aripiprazole relapsed with respect to their symptoms of irritability, compared with 52% of children/adolescents randomised to placebo, for a hazard ratio of 0.57 (95% CI 0.28 to 1.12, 85 children/adolescents, low-quality evidence).All three included trials were supported by Bristol-Myers Squibb (Princeton, NJ) and Otsuka Pharmaceutical Company, Ltd. (Tokyo, Japan), with editorial support provided by Ogilvy Healthworld Medical Education and Bristol-Myers Squibb.
Authors' conclusions: Evidence from two RCTs suggests that aripiprazole can be effective as a short-term medication intervention for some behavioural aspects of ASD in children/adolescents. After a short-term medication intervention with aripiprazole, children/adolescents showed less irritability and hyperactivity and fewer stereotypies (repetitive, purposeless actions). However, notable side effects, such as weight gain, sedation, drooling and tremor, must be considered. One long-term, placebo discontinuation study found that relapse rates did not differ between children/adolescents randomised to continue aripiprazole versus children/adolescents randomised to receive placebo, suggesting that re-evaluation of aripiprazole use after a period of stabilisation in irritability symptoms is warranted. Studies included in this review used criteria from the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) (APA 2000) for ASD diagnosis; however, the diagnostic criteria for ASD changed significantly with release of the fifth edition of the DSM (DSM-5) in 2013 (APA 2013).
Conflict of interest statement
Lauren Hirsch has no conflicts of interest to declare.
Tamara Pringsheim (TP) has received direct financial gain from:
Teva Canada Innovation, on one occasion in 2014, for consulting. TP declares that Teva does not make or market any products relevant to this review.
TP has received indirect financial gain from:
Shire Canada, in 2015. TP received an unrestricted educational grant to create an educational curriculum on assessment and treatment of aggression in youth. TP declares that Shire does not make or market any products relevant to this review.
TP declares that the entities listed below are funding agencies that do not make treatments for ASD or any other disease.
Canadian Institutes of Health Research, for research funding to create a patient decision aid in 2014 and 2015.
Alberta Mental Health Strategic Clinical Network, for research funding on off‐label prescribing of antipsychotics in 2015.
Mathison Centre for Mental Health Research and Education, for research funding for the Canadian Schizophrenia Guidelines in 2015.
Figures


















Update of
-
Aripiprazole for autism spectrum disorders (ASD).Cochrane Database Syst Rev. 2012 May 16;(5):CD009043. doi: 10.1002/14651858.CD009043.pub2. Cochrane Database Syst Rev. 2012. Update in: Cochrane Database Syst Rev. 2016 Jun 26;(6):CD009043. doi: 10.1002/14651858.CD009043.pub3. PMID: 22592735 Updated.
References
References to studies included in this review
Findling 2014 {published data only}
-
- Findling RL, Mankosi R, Timko K, Lears K, McCartney T, McQuade RD, et al. Randomized controlled trial investigating the safety and efficacy of aripiprazole in the long‐term maintenance treatment of pediatric patients with irritability associated with autistic disorder. Journal of Clinical Psychiatry 2014;75(1):22‐30. [PUBMED: 24502859] - PubMed
Marcus 2009 {published data only}
-
- Marcus RN, Owen R, Kame L, Manos G, McQuade RD, Carson WH, et al. A placebo‐controlled, fixed‐dose study of aripiprazole in children and adolescents with irritability associated with autistic disorder. Journal of the American Academy of Child and Adolescent Psychiatry 2009;48(11):1110‐9. [PUBMED: 19797985] - PubMed
Owen 2009 {published data only}
-
- Owen R, Sikich L, Marcus RN, Corey‐Lisle P, Manos G, McQuade RD, et al. Aripiprazole in the treatment of irritability in children and adolescents with autistic disorder. Pediatrics 2009;124(6):1533‐40. [PUBMED: 19948625] - PubMed
References to studies excluded from this review
Aman 2010 {published data only}
-
- Aman M, Kasper W. Line‐item analysis of the Aberrant Behaviour Checklist: results from two studies of aripiprazole in the treatment of irritability associated with autistic disorder. Journal of Child and Adolescent Psychopharmacology 2010;20(5):415‐22. [PUBMED: 20973712] - PubMed
Benton 2011 {published data only}
Blankenship 2010 {published data only}
D'Alessandro 2012 {published data only}
-
- D'Alessandro TMM. Low‐dose Abilify and the Treatment of Disruptive Behaviours in Children with Autism Spectrum Disorders [PhD thesis]. Florida: University of Florida, 2012.
Douglas‐Hall 2011 {published data only}
Erickson 2010 {published data only}
Farmer 2011 {published data only}
Ghanizadeh 2014 {published data only}
-
- Ghanizadeh A, Sahraeizadeh A, Berk M. A head‐to‐head comparison of aripiprazole and risperidone for safety and treating autistic disorders, a randomized double blind clinical trial. Child Psychiatry and Human Development 2014;45(2):185‐92. [PUBMED: 23801256] - PubMed
Huang 2010 {published data only}
-
- Huang SC, Tsai SJ, Yang HJ. Aripiprazole improves social interaction in Taiwanese children with pervasive developmental disorder. Chang Gung Medical Journal 2010;33(2):211‐5. - PubMed
Lewis 2009 {published data only}
-
- Lewis D, Owen R. Efficacy and safety of aripiprazole for the treatment of irritability associated with autistic disorder in children and adolescents (6‐17 years): results from two 8‐week, randomized, double‐blind, placebo controlled trials. Neurology 2009;72(11 Suppl 3):A428.
Maloney 2014 {published data only}
Masi 2009 {published data only}
-
- Masi G, Cosenza A, Millepiedi S, Muratori F, Pari C, Salvadori F. Aripiprazole monotherapy in children and young adolescents with pervasive developmental disorders: a retrospective study. CNS Drugs 2009;23(6):511‐21. - PubMed
Narasimhan 2006 {published data only}
-
- Narasimhan T, Bruce T, BallardJF, Patkar A, Masand P, Rech R. Poster session III: an open label trial of aripiprazole in the treatment of autism and its correlation to whole blood serotonin levels and serotonin transporter (5HTTPLR) function. Neuropsychopharmacology. Proceedings of the American College of Neuropsychopharmacology 45th Annual Meeting; 2006 December 3‐7; Hollywood, Florida. 2006; Vol. 31 (Suppl 1s):S259‐60.
Robb 2011 {published data only}
-
- Robb A, Andersson C, Bellocchio EE, Manos G, Rojas‐Fernandez C, Mathew S, et al. Safety and tolerability of aripiprazole in the treatment of irritability associated with autistic disorder in pediatric subjects (6‐17 years): results from a pooled analysis of 2 studies. Primary Care Companion for CNS Disorders 2011;13(1):e1‐9. [PUBMED: 21731831] - PMC - PubMed
Stigler 2004 {published data only}
-
- Stigler K, Posey D, McDougle CJ. Aripiprazole for maladaptive behaviour in pervasive developmental disorders. Journal of Child and Adolescent Psychopharmacology 2004;14(3):455‐63. [PUBMED: 15650503] - PubMed
Stigler 2006 {published data only}
-
- Stigler KA, Diener JT, Kohn AE, Erickson CA, Posey DJ. McDougle CJ. Poster session II: a prospective open‐label study of aripiprazole in youth with Asperger's disorder and pervasive developmental disorder not otherwise specified. Neuropsychopharmacology. Proceedings of the American College of Neuropsychopharmacology 45th Annual Meeting; 2006 December 3‐7; Hollywood, Florida. 2006; Vol. 31 (Suppl 1s):S194.
Varni 2012 {published data only}
-
- Varni, JW, Handen, BL, Corey‐Lisle P, Guo Z, Manos G, Ammerman DK, et al. Effect of aripiprazole 2 to 15 mg/d on health‐related quality of life in the treatment of irritability associated with autistic disorder in children: a post hoc analysis of two randomized controlled trials. Clinical Therapeutics 2012;34(4):982‐92. [PUBMED: 22444782] - PubMed
Additional references
Aman 1986
-
- Aman M, Singh N. Aberrant Behaviour Checklist: Manual. New York: Slosson Educational Publications, 1986.
Anderson 1989
-
- Anderson LT, Campbell M, Adams P, Small AM, Perry R, Shell J. The effects of haloperidol on discrimination learning and behavioural symptoms in autistic children. Journal of Autism and Developmental Disorders 1989;19(2):227‐39. - PubMed
APA 2000
-
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th Edition. Washington, DC: American Psychiatric Association, 2000.
APA 2013
-
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th Edition. Arlington, VA: American Psyciatric Publishing, 2013.
Barnes 1989
-
- Barnes TR. A rating scale for drug‐induced akathisia. British Journal of Psychiatry 1989;154:672‐6. [PUBMED: 2574607] - PubMed
Baron‐Cohen 2009
-
- Baron‐Cohen S, Scott FJ, Allison C, Williams J, Bolton P, Matthews FE, et al. Prevalence of autism spectrum conditions: UK school based population study. British Journal of Psychiatry 2009;194(6):500‐9. - PubMed
Bobo 2013
-
- Bobo WV, Cooper WO, Stein CM, Olfson M, Graham D, Daugherty J, et al. Antipsychotics and the risk of type 2 diabetes mellitus in children and youth. JAMA Psychiatry 2013;70(10):1067‐75. [PUBMED: 23965896] - PubMed
Campbell 1982
-
- Campbell M, Anderson LT, Small AM, Perry R, Green WH, Caplan R. The effects of haloperidol on learning and behaviour in autistic children. Journal of Autism and Developmental Disorders 1982;12(2):167‐75. [PUBMED: 7174605] - PubMed
Campbell 1997
-
- Campbell M, Armenteros JL, Malone RP, Adams PB, Eisenberg ZW, Overall JE. Neuroleptic‐related dyskinesias in autistic children: a prospective, longitudinal study. Journal of the American Academy of Child and Adolescent Psychiatry 1997;36(6):835‐43. [PUBMED: 9183140] - PubMed
CDC 2014
-
- Centers for Disease Control and Prevention. Community Report from the Autism and Developmental Disabilities Monitoring (ADDM) Network: a snapshot of autism spectrum disorder among 8‐year‐old children in multiple communities across the United States in 2010. http://www.cdc.gov/ncbddd/autism/documents/community_report_autism.pdf (accessed 4 September 2015).
Correll 2009
Elbourne 2002
-
- Elbourne DR, Altman DG, Higgins JP, Curtin F, Worthington HV, Vail A. Meta‐analysis involving cross‐over trials: methodological issues. International Journal of Epidemiology 2002;31(1):140‐9. [PUBMED: 11914310] - PubMed
Elsabbagh 2012
FDA 2010
-
- Food, Drug Administration. Label and approval history: ability. www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm?fuseraction=Sea... (accessed 28 February 2010).
Findling 2008
-
- Findling RL, Robb A, Nyilas M, Forbes RA, Jin N, Ivanova S, et al. A multiple‐center, randomized, double‐blind, placebo‐controlled study of oral aripiprazole for treatment of adolescents with schizophrenia. American Journal of Psychiatry 2008;165(11):1432‐41. [PUBMED: 18765484] - PubMed
Findling 2009
-
- Findling RL, Nyilas M, Forbes RA, McQuade RD, Jin N, Iwamoto T, et al. Acute treatment of pediatric bipolar I disorder, manic or mixed episode, with aripiprazole: a randomized, double‐blind, placebo‐controlled study. Journal of Clinical Psychiatry 2009;70(10):1441‐51. [PUBMED: 19906348] - PubMed
Goodman 1989
-
- Goodman WK, Price LH, Rasmussen SA, Mazure C, Fleischmann RL, Hill CL, et al. The Yale‐Brown Obsessive Compulsive Scale. I. Development, use, and reliability. Archives of General Psychiatry 1989;46(11):1006‐11. [PUBMED: 2684084] - PubMed
Goodnick 2002
-
- Goodnick PJ, Jerry JM. Aripiprazole: profile on efficacy and safety. Expert Opinion on Pharmacotherapy 2002;3(12):1773‐81. [PUBMED: 12472374] - PubMed
Guy 1976
-
- Guy W. Early Clinical Drug Evaluation Unit (ECDEU) Assessment Manual for Psychopharmacology. National Institute Mental Health. Bethesda, MD: National Institute of Mental Health, 1976.
Guyatt 2011
-
- Guyatt G, Oxman AD, Aki EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction ‐ GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383‐94. - PubMed
Higgins 2011a
-
- Higgins JPT, Altman DG, Sterne J on behalf of the Cochrane Statistical Methods Group and the Cochrane Bias Methods Group. Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Intervention Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from http://handbook.cochrane.org.
Higgins 2011b
-
- Higgins JPT, Deeks JJ, Altman DG on behalf of the Cochrane Statistical Methods Group. Chapter 16: Special topics in statistics. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from http://handbook.cochrane.org.
Higgins 2011c
-
- Deeks JJ, Higgins JPT, Altman DG on behalf of the Cochrane Statistical Methods Group. Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Jesner 2009
Kanner 1943
-
- Kanner L. Autistic disturbances of affective contact. Nervous Child 1943;2:217‐50. - PubMed
Kogan 2009
-
- Kogan MD, Blumberg SJ, Schieve LA, Boyle CA, Perrin JM, Ghandour RM, et al. Prevalence of parent‐reported diagnosis of autism spectrum disorders among children in the US, 2007. Pediatrics 2009;124(5):1395‐403. - PubMed
Komossa 2009
Lecavalier 2006
-
- Lecavalier L. Behavioral and emotional problems in young people with pervasive developmental disorders: relative prevalence, effects of subject characteristics, and empirical classification. Journal of Autism and Developmental Disorders 2006;36(8):1101‐14. [PUBMED: 16897387] - PubMed
Lord 1994
-
- Lord C, Rutter M, Couteur A. Autism Diagnostic Interview ‐ Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism and Developmental Disorders 1994;24(5):659‐85. - PubMed
Meyers 2007
-
- Myers S, Johnson C, Council on Children with Disabilities. Management of children with autism spectrum disorders. Pediatrics 2007;120(5):1162‐82. - PubMed
Posey 2008
RevMan 2014 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Scahill 1997
-
- Scahill L, Riddle M, McSwiggin‐Hardin M, Ort SI, King RA, Goodman WK, et al. Children's Yale‐Brown Obsessive Compulsive Scale: reliability and validity. Journal of the American Academy of Child and Adolescent Psychiatry 1997;36(6):844‐52. [PUBMED: 9183141] - PubMed
Shapiro 2003
-
- Shapiro DA, Renock S, Arrington E, Chiodo LA, Liu LX, Sibley DR, et al. Aripiprazole, a novel atypical antipsychotic drug with a unique and robust pharmacology. Neuropsychopharmacology 2003;28(8):1400‐11. [PUBMED: 12784105] - PubMed
Simonoff 2008
-
- Simonoff E, Pickles A, Charman T, Chandler S, Loucas T, Baird G. Psychiatric disorders in children with autism spectrum disorders: prevalence, comorbidity and associated factors in a population derived sample. Journal of the American Academy of Child and Adolescent Psychiatry 2008;47(8):921‐9. [PUBMED: 18645422] - PubMed
Simpson 1970
-
- Simpson GM, Agnus JWS. A ratings scale for extrapyramidal side effects. Acta Psychiatrica Scandinavica 1970;45(S212):11‐9. - PubMed
Stigler 2009
-
- Stigler KA, Diener, JT, Kohn AE, Li L, Erickson CA, Posey DJ, et al. Aripiprazole in pervasive development disorder not otherwise specified and Asperger's disorder: a 14‐week, prospective, open‐label study. Journal of Child and Adolescent Psychopharmacology 2009;19(3):265‐74. [PUBMED: 19519261] - PMC - PubMed
Varni 1999
-
- Varni JW, Seid M, Rode CA. The PedsQL: measurement model for the pediatric quality of life inventory. Medical Care 1999;37(2):126‐39. [PUBMED: 10024117] - PubMed
Webster 1968
-
- Webster DD. Critical analysis of the disability in Parkinson's disease. Modern Treatment 1968;5(2):279‐81. [PUBMED: 5655944] - PubMed
WHO 1994
-
- World Health Organization. International Statistical Classification of Diseases and Related Health Problems, 10th Revision. Vol. 1‐3, Geneva: World Health Organization, 1994.
Williams 2006
Yudofsky 1986
-
- Yudofsky SC, Silver JM, Jackson W, Endicott J, Williams D. The Overt Aggression Scale for the objective rating of verbal and physical aggression. American Journal of Psychiatry 1986;143(1):35‐9. [PUBMED: 3942284] - PubMed
References to other published versions of this review
Ching 2011
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

