NRF2 and glutathione are key resistance mediators to temozolomide in glioma and melanoma cells
- PMID: 27344172
- PMCID: PMC5217002
- DOI: 10.18632/oncotarget.10129
NRF2 and glutathione are key resistance mediators to temozolomide in glioma and melanoma cells
Abstract
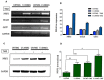
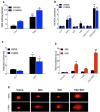
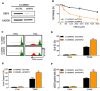
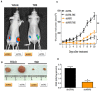
Cancer is a leading cause of death worldwide, and while great advances have been made particularly in chemotherapy, many types of cancer still present a dismal prognosis. In the case of glioma, temozolomide (TMZ) is the main option for treatment, but it has limited success due to drug resistance. While this resistance is usually associated to DNA repair mechanisms, in this work we demonstrate that oxidative stress plays an important role. We showed that upon TMZ treatment there is an induction of the nuclear factor erythroid 2-related factor 2 (NRF2), which is the main antioxidant transcription factor regulator in human cells. This is accompanied by an enhancement of glutathione (GSH) concentration in the tumor cells. The effectiveness of this pathway was proven by silencing NFR2, which greatly enhanced cell death upon TMZ treatment both in vitro and in vivo. Also, higher DNA damage and induced cell death was observed by combining BSO - a GSH inhibitor - with TMZ. Similar effects were also observed using in vitro and in vivo models of melanoma, thus possibly indicating that GSH has a decisive role in TMZ resistance in a wider range of tumors. Thus, a combined regimen of BSO and TMZ configures an interesting therapeutic alternative for fighting both glioma and melanoma.
Keywords: NRF2; glioma; melanoma; resistance; temozolomide.
Conflict of interest statement
Conflicts of Interest: None declared.
Figures

Similar articles
-
NVP-BEZ235, a novel dual PI3K-mTOR inhibitor displays anti-glioma activity and reduces chemoresistance to temozolomide in human glioma cells.Cancer Lett. 2015 Oct 10;367(1):58-68. doi: 10.1016/j.canlet.2015.07.007. Epub 2015 Jul 15. Cancer Lett. 2015. PMID: 26188279
-
Glutathione depletion sensitizes cisplatin- and temozolomide-resistant glioma cells in vitro and in vivo.Cell Death Dis. 2014 Oct 30;5(10):e1505. doi: 10.1038/cddis.2014.465. Cell Death Dis. 2014. PMID: 25356874 Free PMC article.
-
Inhibition of DNA-repair genes Ercc1 and Mgmt enhances temozolomide efficacy in gliomas treatment: a pre-clinical study.Oncotarget. 2015 Oct 6;6(30):29456-68. doi: 10.18632/oncotarget.4909. Oncotarget. 2015. PMID: 26336131 Free PMC article.
-
A novel approach to overcome temozolomide resistance in glioma and melanoma: Inactivation of MGMT by gene therapy.Biochem Biophys Res Commun. 2011 Mar 18;406(3):311-4. doi: 10.1016/j.bbrc.2011.02.042. Epub 2011 Feb 15. Biochem Biophys Res Commun. 2011. PMID: 21329652 Review.
-
Temozolomide in malignant gliomas: current use and future targets.Cancer Chemother Pharmacol. 2009 Sep;64(4):647-55. doi: 10.1007/s00280-009-1050-5. Epub 2009 Jun 19. Cancer Chemother Pharmacol. 2009. PMID: 19543728 Review.
Cited by
-
Exploring the multifaceted role of NRF2 in brain physiology and cancer: A comprehensive review.Neurooncol Adv. 2023 Dec 23;6(1):vdad160. doi: 10.1093/noajnl/vdad160. eCollection 2024 Jan-Dec. Neurooncol Adv. 2023. PMID: 38221979 Free PMC article. Review.
-
DNA repair pathways and cisplatin resistance: an intimate relationship.Clinics (Sao Paulo). 2018 Sep 6;73(suppl 1):e478s. doi: 10.6061/clinics/2018/e478s. Clinics (Sao Paulo). 2018. PMID: 30208165 Free PMC article. Review.
-
Adjusting the Molecular Clock: The Importance of Circadian Rhythms in the Development of Glioblastomas and Its Intervention as a Therapeutic Strategy.Int J Mol Sci. 2021 Aug 1;22(15):8289. doi: 10.3390/ijms22158289. Int J Mol Sci. 2021. PMID: 34361055 Free PMC article. Review.
-
Cold Atmospheric Plasma Increases Temozolomide Sensitivity of Three-Dimensional Glioblastoma Spheroids via Oxidative Stress-Mediated DNA Damage.Cancers (Basel). 2021 Apr 8;13(8):1780. doi: 10.3390/cancers13081780. Cancers (Basel). 2021. PMID: 33917880 Free PMC article.
-
Exploring the Use of Cold Atmospheric Plasma to Overcome Drug Resistance in Cancer.Biomedicines. 2023 Jan 14;11(1):208. doi: 10.3390/biomedicines11010208. Biomedicines. 2023. PMID: 36672716 Free PMC article. Review.
References
-
- Stupp R, Tonn JC, Brada M, Pentheroudakis G. High-grade malignant glioma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2010;2:190–193. - PubMed
-
- Wen PY, Kesari S. Malignant gliomas in adults. N. Engl. J. Med. 2008;359:492–507. - PubMed
-
- van den Bent MJ, Hegi ME, Stupp R. Recent developments in the use of chemotherapy in brain tumours. Eur. J. Cancer. 2006;42:582–588. - PubMed
-
- Fonkem E, Uhlmann E, Floyd EJ, Mahadevan SR, Kasper A, Eton E, Wong ET. Melanoma brain metastasis: overview of current management and emerging targeted therapies. Expert Rev. Neurother. 2012;12:1207–1215. - PubMed
-
- Schadendorf D, Fisher D, Garbe DE, Gershenwald C, Grob JE, Halpern J, Herlyn A, Marchetti M, McArthur MA, Ribas G, Roesch A, Hauschild A. Melanoma. Nat. Rev. Dis. Prim. 2015:15003. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

