Comparative biology of decellularized lung matrix: Implications of species mismatch in regenerative medicine
- PMID: 27344365
- PMCID: PMC4939101
- DOI: 10.1016/j.biomaterials.2016.06.025
Comparative biology of decellularized lung matrix: Implications of species mismatch in regenerative medicine
Abstract
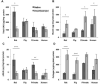
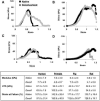
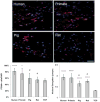
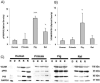
Lung engineering is a promising technology, relying on re-seeding of either human or xenographic decellularized matrices with patient-derived pulmonary cells. Little is known about the species-specificity of decellularization in various models of lung regeneration, or if species dependent cell-matrix interactions exist within these systems. Therefore decellularized scaffolds were produced from rat, pig, primate and human lungs, and assessed by measuring residual DNA, mechanical properties, and key matrix proteins (collagen, elastin, glycosaminoglycans). To study intrinsic matrix biologic cues, human endothelial cells were seeded onto acellular slices and analyzed for markers of cell health and inflammation. Despite similar levels of collagen after decellularization, human and primate lungs were stiffer, contained more elastin, and retained fewer glycosaminoglycans than pig or rat lung scaffolds. Human endothelial cells seeded onto human and primate lung tissue demonstrated less expression of vascular cell adhesion molecule and activation of nuclear factor-κB compared to those seeded onto rodent or porcine tissue. Adhesion of endothelial cells was markedly enhanced on human and primate tissues. Our work suggests that species-dependent biologic cues intrinsic to lung extracellular matrix could have profound effects on attempts at lung regeneration.
Keywords: Bioactivity; Decellularization; Extracellular matrix; Lung tissue engineering.
Copyright © 2016 Elsevier Ltd. All rights reserved.
Figures



References
-
- Yusen RD, Edwards LB, Kucheryavaya AY, Benden C, Dipchand AI, Dobbels F, Goldfarb SB, Levvey BJ, Lund LH, Meiser B, Stehlik J International Society for H, Lung T. The registry of the International Society for Heart and Lung Transplantation: thirty-first adult lung and heart-lung transplant report--2014; focus theme: retransplantation. J Heart Lung Transplant. 2014;33:1009–1024. - PubMed
-
- Ott HC, Clippinger B, Conrad C, Schuetz C, Pomerantseva I, Ikonomou L, Kotton D, Vacanti JP. Regeneration and orthotopic transplantation of a bioartificial lung. Nat Med. 2010;16:927–933. - PubMed
-
- Nichols JE, Niles J, Riddle M, Vargas G, Schilagard T, Ma L, Edward K, La Francesca S, Sakamoto J, Vega S, Ogadegbe M, Mlcak R, Deyo D, Woodson L, McQuitty C, Lick S, Beckles D, Melo E, Cortiella J. Production and assessment of decellularized pig and human lung scaffolds. Tissue Eng Part A. 2013;19:2045–2062. - PMC - PubMed
-
- Bonvillain RW, Danchuk S, Sullivan DE, Betancourt AM, Semon JA, Eagle ME, Mayeux JP, Gregory AN, Wang G, Townley IK, Borg ZD, Weiss DJ, Bunnell BA. A nonhuman primate model of lung regeneration: detergent-mediated decellularization and initial in vitro recellularization with mesenchymal stem cells. Tissue Eng Part A. 2012;18:2437–2452. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

