Discovery and development of natural product oridonin-inspired anticancer agents
- PMID: 27344488
- PMCID: PMC5003635
- DOI: 10.1016/j.ejmech.2016.06.015
Discovery and development of natural product oridonin-inspired anticancer agents
Abstract
Natural products have historically been, and continue to be, an invaluable source for the discovery of various therapeutic agents. Oridonin, a natural diterpenoid widely applied in traditional Chinese medicines, exhibits a broad range of biological effects including anticancer and anti-inflammatory activities. To further improve its potency, aqueous solubility and bioavailability, the oridonin template serves as an exciting platform for drug discovery to yield better candidates with unique targets and enhanced drug properties. A number of oridonin derivatives (e.g. HAO472) have been designed and synthesized, and have contributed to substantial progress in the identification of new agents and relevant molecular mechanistic studies toward the treatment of human cancers and other diseases. This review summarizes the recent advances in medicinal chemistry on the explorations of novel oridonin analogues as potential anticancer therapeutics, and provides a detailed discussion of future directions for the development and progression of this class of molecules into the clinic.
Keywords: Anticancer agents; Chemical biology; Diterpenoids; Drug discovery; Natural product; Oridonin.
Copyright © 2016 Elsevier Masson SAS. All rights reserved.
Conflict of interest statement
The authors confirm that this article content has no conflicts of interest.
Figures

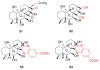
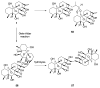
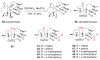
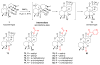


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

