Peptide drugs accelerate BMP-2-induced calvarial bone regeneration and stimulate osteoblast differentiation through mTORC1 signaling
- PMID: 27345003
- PMCID: PMC5094554
- DOI: 10.1002/bies.201600104
Peptide drugs accelerate BMP-2-induced calvarial bone regeneration and stimulate osteoblast differentiation through mTORC1 signaling
Abstract
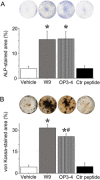
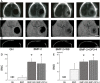
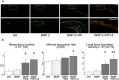
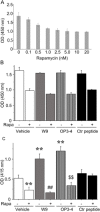
Both W9 and OP3-4 were known to bind the receptor activator of NF-κB ligand (RANKL), inhibiting osteoclastogenesis. Recently, both peptides were shown to stimulate osteoblast differentiation; however, the mechanism underlying the activity of these peptides remains to be clarified. A primary osteoblast culture showed that rapamycin, an mTORC1 inhibitor, which was recently demonstrated to be an important serine/threonine kinase for bone formation, inhibited the peptide-induced alkaline phosphatase activity. Furthermore, both peptides promoted the phosphorylation of Akt and S6K1, an upstream molecule of mTORC1 and the effector molecule of mTORC1, respectively. In the in vivo calvarial defect model, W9 and OP3-4 accelerated BMP-2-induced bone formation to a similar extent, which was confirmed by histomorphometric analyses using fluorescence images of undecalcified sections. Our data suggest that these RANKL-binding peptides could stimulate the mTORC1 activity, which might play a role in the acceleration of BMP-2-induced bone regeneration by the RANKL-binding peptides.
Keywords: BMP-2; bone regeneration; histomorphometry; mTORC1; osteoblast differentiation; peptide therapeutics; rapamycin.
© 2016 The Authors. Bioessays published by WILEY Periodicals, Inc.
Figures

References
-
- Feeley BT, Krenek L, Liu N, Hsu WK, et al. 2006. Overexpression of noggin inhibits BMP‐mediated growth of osteolytic prostate cancer lesions. Bone 38: 154–66. - PubMed
-
- Carreira AC, Lojudice FH, Halcsik E, Navarro RD, et al. 2014. Bone morphogenetic proteins: facts, challenges, and future perspectives. J Dent Res 93: 335–45. - PubMed
-
- Yun JI, Wikesjo UM, Borke JL, Bisch FC, et al. 2010. Effect of systemic parathyroid hormone (1‐34) and a beta‐tricalcium phosphate biomaterial on local bone formation in a critical‐size rat calvarial defect model. J Clin Periodontol 37: 419–26. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

