A previously unrecognized role of C3a in proteinuric progressive nephropathy
- PMID: 27345360
- PMCID: PMC4921969
- DOI: 10.1038/srep28445
A previously unrecognized role of C3a in proteinuric progressive nephropathy
Abstract
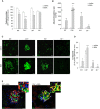
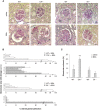
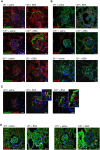
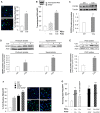
Podocyte loss is the initial event in the development of glomerulosclerosis, the structural hallmark of progressive proteinuric nephropathies. Understanding mechanisms underlying glomerular injury is the key challenge for identifying novel therapeutic targets. In mice with protein-overload induced by bovine serum albumin (BSA), we evaluated whether the alternative pathway (AP) of complement mediated podocyte depletion and podocyte-dependent parietal epithelial cell (PEC) activation causing glomerulosclerosis. Factor H (Cfh(-/-)) or factor B-deficient mice were studied in comparison with wild-type (WT) littermates. WT+BSA mice showed podocyte depletion accompanied by glomerular complement C3 and C3a deposits, PEC migration to capillary tuft, proliferation, and glomerulosclerosis. These changes were more prominent in Cfh(-/-) +BSA mice. The pathogenic role of AP was documented by data that factor B deficiency preserved glomerular integrity. In protein-overload mice, PEC dysregulation was associated with upregulation of CXCR4 and GDNF/c-Ret axis. In vitro studies provided additional evidence of a direct action of C3a on proliferation and CXCR4-related migration of PECs. These effects were enhanced by podocyte-derived GDNF. In patients with proteinuric nephropathy, glomerular C3/C3a paralleled PEC activation, CXCR4 and GDNF upregulation. These results indicate that mechanistically uncontrolled AP complement activation is not dispensable for podocyte-dependent PEC activation resulting in glomerulosclerosis.
Figures

Similar articles
-
mTOR-mediated podocyte hypertrophy regulates glomerular integrity in mice and humans.JCI Insight. 2019 Sep 19;4(18):e99271. doi: 10.1172/jci.insight.99271. JCI Insight. 2019. PMID: 31534053 Free PMC article.
-
Complement C5a and C5a receptor 1 mediates glomerular damage in focal segmental glomerulosclerosis.Clin Immunol. 2025 Apr;273:110459. doi: 10.1016/j.clim.2025.110459. Epub 2025 Feb 19. Clin Immunol. 2025. PMID: 39984108
-
Glomerular parietal epithelial cells contribute to adult podocyte regeneration in experimental focal segmental glomerulosclerosis.Kidney Int. 2015 Nov;88(5):999-1012. doi: 10.1038/ki.2015.152. Epub 2015 May 20. Kidney Int. 2015. PMID: 25993321 Free PMC article.
-
Podocyte injury in focal segmental glomerulosclerosis: Lessons from animal models (a play in five acts).Kidney Int. 2008 Feb;73(4):399-406. doi: 10.1038/sj.ki.5002655. Epub 2007 Nov 7. Kidney Int. 2008. PMID: 17989648 Review.
-
Parietal epithelial cells and podocytes in glomerular diseases.Semin Nephrol. 2012 Jul;32(4):357-67. doi: 10.1016/j.semnephrol.2012.06.007. Semin Nephrol. 2012. PMID: 22958490 Review.
Cited by
-
A novel approach to immunoapheresis of C3a/C3 and proteomic identification of associates.PeerJ. 2019 Dec 16;7:e8218. doi: 10.7717/peerj.8218. eCollection 2019. PeerJ. 2019. PMID: 31871840 Free PMC article.
-
Complement Activation Contributes to the Pathophysiology of Shiga Toxin-Associated Hemolytic Uremic Syndrome.Microorganisms. 2019 Jan 10;7(1):15. doi: 10.3390/microorganisms7010015. Microorganisms. 2019. PMID: 30634669 Free PMC article. Review.
-
The MFHR1 Fusion Protein Is a Novel Synthetic Multitarget Complement Inhibitor with Therapeutic Potential.J Am Soc Nephrol. 2018 Apr;29(4):1141-1153. doi: 10.1681/ASN.2017070738. Epub 2018 Jan 15. J Am Soc Nephrol. 2018. PMID: 29335241 Free PMC article.
-
Loss of decay-accelerating factor triggers podocyte injury and glomerulosclerosis.J Exp Med. 2020 Sep 7;217(9):e20191699. doi: 10.1084/jem.20191699. J Exp Med. 2020. PMID: 32717081 Free PMC article.
-
SGLT2 inhibitor dapagliflozin limits podocyte damage in proteinuric nondiabetic nephropathy.JCI Insight. 2018 Aug 9;3(15):e98720. doi: 10.1172/jci.insight.98720. eCollection 2018 Aug 9. JCI Insight. 2018. PMID: 30089717 Free PMC article.
References
-
- D’Agati V. D., Kaskel F. J. & Falk R. J. Focal segmental glomerulosclerosis. N Engl J Med 365, 2398–2411 (2011). - PubMed
-
- Satirapoj B., Nast C. C. & Adler S. G. Novel insights into the relationship between glomerular pathology and progressive kidney disease. Adv Chronic Kidney Dis 19, 93–100 (2012). - PubMed
-
- Zoja C., Abbate M. & Remuzzi G. Progression of renal injury toward interstitial inflammation and glomerular sclerosis is dependent on abnormal protein filtration. Nephrol Dial Transplant 30, 706–712 (2015). - PubMed
-
- Kim Y. H. et al.. Podocyte depletion and glomerulosclerosis have a direct relationship in the PAN-treated rat. Kidney Int 60, 957–968 (2001). - PubMed
-
- Kriz W. Podocyte is the major culprit accounting for the progression of chronic renal disease. Microsc Res Tech 57, 189–195 (2002). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

