Sugar Synthesis from CO2 in Escherichia coli
- PMID: 27345370
- PMCID: PMC4930481
- DOI: 10.1016/j.cell.2016.05.064
Sugar Synthesis from CO2 in Escherichia coli
Abstract
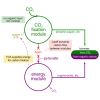
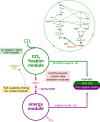
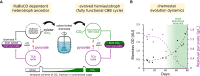
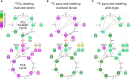
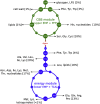
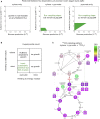
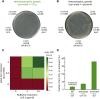
Can a heterotrophic organism be evolved to synthesize biomass from CO2 directly? So far, non-native carbon fixation in which biomass precursors are synthesized solely from CO2 has remained an elusive grand challenge. Here, we demonstrate how a combination of rational metabolic rewiring, recombinant expression, and laboratory evolution has led to the biosynthesis of sugars and other major biomass constituents by a fully functional Calvin-Benson-Bassham (CBB) cycle in E. coli. In the evolved bacteria, carbon fixation is performed via a non-native CBB cycle, while reducing power and energy are obtained by oxidizing a supplied organic compound (e.g., pyruvate). Genome sequencing reveals that mutations in flux branchpoints, connecting the non-native CBB cycle to biosynthetic pathways, are essential for this phenotype. The successful evolution of a non-native carbon fixation pathway, though not yet resulting in net carbon gain, strikingly demonstrates the capacity for rapid trophic-mode evolution of metabolism applicable to biotechnology. PAPERCLIP.
Copyright © 2016 The Author(s). Published by Elsevier Inc. All rights reserved.
Figures


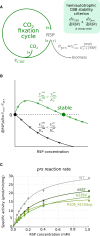
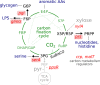

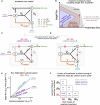
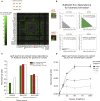

Comment in
-
E. coli bacteria engineered to eat carbon dioxide.Nature. 2019 Dec;576(7785):19-20. doi: 10.1038/d41586-019-03679-x. Nature. 2019. PMID: 31796902 No abstract available.
References
-
- Bar-Even A., Noor E., Milo R. A survey of carbon fixation pathways through a quantitative lens. J. Exp. Bot. 2012;63:2325–2342. - PubMed
-
- Barrick J.E., Yu D.S., Yoon S.H., Jeong H., Oh T.K., Schneider D., Lenski R.E., Kim J.F. Genome evolution and adaptation in a long-term experiment with Escherichia coli. Nature. 2009;461:1243–1247. - PubMed
-
- Bassham J.A., Benson A.A., Kay L.D., Harris A.Z., Wilson A.T., Calvin M. The path of carbon in photosynthesis. XXI. The cyclic regeneration of carbon dioxide acceptor1. J. Am. Chem. Soc. 1954;76:1760–1770.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

