Netrin-G1 regulates fear-like and anxiety-like behaviors in dissociable neural circuits
- PMID: 27345935
- PMCID: PMC4921862
- DOI: 10.1038/srep28750
Netrin-G1 regulates fear-like and anxiety-like behaviors in dissociable neural circuits
Abstract
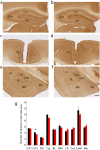
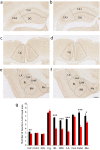
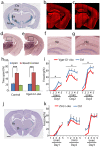
In vertebrate mammals, distributed neural circuits in the brain are involved in emotion-related behavior. Netrin-G1 is a glycosyl-phosphatidylinositol-anchored synaptic adhesion molecule whose deficiency results in impaired fear-like and anxiety-like behaviors under specific circumstances. To understand the cell type and circuit specificity of these responses, we generated netrin-G1 conditional knockout mice with loss of expression in cortical excitatory neurons, inhibitory neurons, or thalamic neurons. Genetic deletion of netrin-G1 in cortical excitatory neurons resulted in altered anxiety-like behavior, but intact fear-like behavior, whereas loss of netrin-G1 in inhibitory neurons resulted in attenuated fear-like behavior, but intact anxiety-like behavior. These data indicate a remarkable double dissociation of fear-like and anxiety-like behaviors involving netrin-G1 in excitatory and inhibitory neurons, respectively. Our findings support a crucial role for netrin-G1 in dissociable neural circuits for the modulation of emotion-related behaviors, and provide genetic models for investigating the mechanisms underlying the dissociation. The results also suggest the involvement of glycosyl-phosphatidylinositol-anchored synaptic adhesion molecules in the development and pathogenesis of emotion-related behavior.
Figures



Similar articles
-
Netrin-G1 Regulates Microglial Accumulation along Axons and Supports the Survival of Layer V Neurons in the Postnatal Mouse Brain.Cell Rep. 2020 Apr 28;31(4):107580. doi: 10.1016/j.celrep.2020.107580. Cell Rep. 2020. PMID: 32348754
-
Diversification of behavior and postsynaptic properties by netrin-G presynaptic adhesion family proteins.Mol Brain. 2016 Jan 8;9:6. doi: 10.1186/s13041-016-0187-5. Mol Brain. 2016. PMID: 26746425 Free PMC article.
-
Netrin-G1: a novel glycosyl phosphatidylinositol-linked mammalian netrin that is functionally divergent from classical netrins.J Neurosci. 2000 Sep 1;20(17):6540-50. doi: 10.1523/JNEUROSCI.20-17-06540.2000. J Neurosci. 2000. PMID: 10964959 Free PMC article.
-
Neuropeptide S: a transmitter system in the brain regulating fear and anxiety.Neuropharmacology. 2010 Jan;58(1):29-34. doi: 10.1016/j.neuropharm.2009.06.001. Epub 2009 Jun 10. Neuropharmacology. 2010. PMID: 19523478 Free PMC article. Review.
-
Fear, anxiety, and control in the zebrafish.Dev Neurobiol. 2012 Mar;72(3):395-403. doi: 10.1002/dneu.20873. Dev Neurobiol. 2012. PMID: 22328274 Review.
Cited by
-
A single-cell multi-omic atlas spanning the adult rhesus macaque brain.Sci Adv. 2023 Oct 13;9(41):eadh1914. doi: 10.1126/sciadv.adh1914. Epub 2023 Oct 12. Sci Adv. 2023. PMID: 37824616 Free PMC article.
-
Altered genome-wide hippocampal gene expression profiles following early life lead exposure and their potential for reversal by environmental enrichment.Sci Rep. 2022 Jul 25;12(1):11937. doi: 10.1038/s41598-022-15861-9. Sci Rep. 2022. PMID: 35879375 Free PMC article.
-
A BRIEF INTRODUCTION TO THE NEUROGENETICS OF COGNITION-EMOTION INTERACTIONS.Curr Opin Behav Sci. 2018 Feb;19:50-54. doi: 10.1016/j.cobeha.2017.09.014. Epub 2017 Oct 15. Curr Opin Behav Sci. 2018. PMID: 29399606 Free PMC article.
-
Homozygous Missense Variants in NTNG2, Encoding a Presynaptic Netrin-G2 Adhesion Protein, Lead to a Distinct Neurodevelopmental Disorder.Am J Hum Genet. 2019 Nov 7;105(5):1048-1056. doi: 10.1016/j.ajhg.2019.09.025. Epub 2019 Oct 24. Am J Hum Genet. 2019. PMID: 31668703 Free PMC article.
-
Abnormal ER quality control of neural GPI-anchored proteins via dysfunction in ER export processing in the frontal cortex of elderly subjects with schizophrenia.Transl Psychiatry. 2019 Jan 16;9(1):6. doi: 10.1038/s41398-018-0359-4. Transl Psychiatry. 2019. PMID: 30664618 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

