Mitochondria, the Cell Cycle, and the Origin of Sex via a Syncytial Eukaryote Common Ancestor
- PMID: 27345956
- PMCID: PMC5390555
- DOI: 10.1093/gbe/evw136
Mitochondria, the Cell Cycle, and the Origin of Sex via a Syncytial Eukaryote Common Ancestor
Abstract
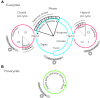
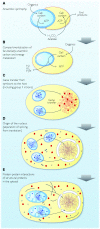
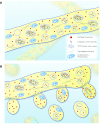
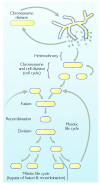
Theories for the origin of sex traditionally start with an asexual mitosing cell and add recombination, thereby deriving meiosis from mitosis. Though sex was clearly present in the eukaryote common ancestor, the order of events linking the origin of sex and the origin of mitosis is unknown. Here, we present an evolutionary inference for the origin of sex starting with a bacterial ancestor of mitochondria in the cytosol of its archaeal host. We posit that symbiotic association led to the origin of mitochondria and gene transfer to host's genome, generating a nucleus and a dedicated translational compartment, the eukaryotic cytosol, in which-by virtue of mitochondria-metabolic energy was not limiting. Spontaneous protein aggregation (monomer polymerization) and Adenosine Tri-phosphate (ATP)-dependent macromolecular movement in the cytosol thereby became selectable, giving rise to continuous microtubule-dependent chromosome separation (reduction division). We propose that eukaryotic chromosome division arose in a filamentous, syncytial, multinucleated ancestor, in which nuclei with insufficient chromosome numbers could complement each other through mRNA in the cytosol and generate new chromosome combinations through karyogamy. A syncytial (or coenocytic, a synonym) eukaryote ancestor, or Coeca, would account for the observation that the process of eukaryotic chromosome separation is more conserved than the process of eukaryotic cell division. The first progeny of such a syncytial ancestor were likely equivalent to meiospores, released into the environment by the host's vesicle secretion machinery. The natural ability of archaea (the host) to fuse and recombine brought forth reciprocal recombination among fusing (syngamy and karyogamy) progeny-sex-in an ancestrally meiotic cell cycle, from which the simpler haploid and diploid mitotic cell cycles arose. The origin of eukaryotes was the origin of vertical lineage inheritance, and sex was required to keep vertically evolving lineages viable by rescuing the incipient eukaryotic lineage from Muller's ratchet. The origin of mitochondria was, in this view, the decisive incident that precipitated symbiosis-specific cell biological problems, the solutions to which were the salient features that distinguish eukaryotes from prokaryotes: A nuclear membrane, energetically affordable ATP-dependent protein-protein interactions in the cytosol, and a cell cycle involving reduction division and reciprocal recombination (sex).
Keywords: alternation of generations; chromosome segregation; coeocytic; endomembrane system; eukaryotes; karyogamy; meiosis; mitosis; origin; reduction division; syngamy.
© The Author(s) 2016. Published by Oxford University Press on behalf of the Society for Molecular Biology and Evolution.
Figures
Comment in
-
Sex First-A New View of the Origins of Eukaryotes.Genome Biol Evol. 2017 Apr 1;9(4):902-903. doi: 10.1093/gbe/evw183. Genome Biol Evol. 2017. PMID: 28423160 Free PMC article. No abstract available.
References
-
- Allen JF. 1996. Separate sexes and the mitochondrial theory of ageing. J Theor Biol. 180:135–140. - PubMed
-
- Bakker EP, Van Dam K. 1974. The movement of monocarboxylic acids across phospholipid membranes: evidence for an exchange diffusion between pyruvate and other monocarboxylate ions. Biochim Biophys Acta–Biomembranes. 339:285–289. - PubMed
-
- Beech PL., et al. 2000. Mitochondrial FtsZ in a chromophyte alga. Science 287:1276–1279. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

