Involvement of Cl(-)/HCO3(-) exchanger SLC26A3 and SLC26A6 in preimplantation embryo cleavage
- PMID: 27346053
- PMCID: PMC4921817
- DOI: 10.1038/srep28402
Involvement of Cl(-)/HCO3(-) exchanger SLC26A3 and SLC26A6 in preimplantation embryo cleavage
Abstract
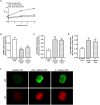
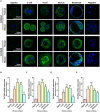
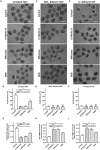
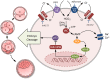
Bicarbonate (HCO3(-)) is essential for preimplantation embryo development. However, the mechanism underlying the HCO3(-) transport into the embryo remains elusive. In the present study, we examined the possible involvement of Cl(-)/HCO3(-) exchanger in mediating HCO3(-) transport into the embryo. Our results showed that depletion of extracellular Cl(-), even in the presence of HCO3(-), suppressed embryo cleavage in a concentration-dependent manner. Cleavage-associated HCO3(-)-dependent events, including increase of intracellular pH, upregulation of miR-125b and downregulation of p53, also required Cl(-). We further showed that Cl(-)/HCO3(-) exchanger solute carrier family 26 (SLC26) A3 and A6 were expressed at 2-cell through blastocyst stage. Blocking individual exchanger's activity by inhibitors or gene knockdown differentially decreased embryo cleavage and inhibited HCO3(-)-dependent events, while inhibiting/knocking down both produced an additive effect to an extent similar to that observed when CFTR was inhibited. These results indicate the involvement of SLC26A3 and A6 in transporting HCO3(-) essential for embryo cleavage, possibly working in concert with CFTR through a Cl(-) recycling pathway. The present study sheds light into our understanding of molecular mechanisms regulating embryo cleavage by the female reproductive tract.
Figures


Similar articles
-
Cl- is required for HCO3- entry necessary for sperm capacitation in guinea pig: involvement of a Cl-/HCO3- exchanger (SLC26A3) and CFTR.Biol Reprod. 2009 Jan;80(1):115-23. doi: 10.1095/biolreprod.108.068528. Epub 2008 Sep 10. Biol Reprod. 2009. PMID: 18784352
-
Cystic fibrosis transmembrane conductance regulator and SLC26 transporters in HCO₃⁻ secretion by pancreatic duct cells.Sheng Li Xue Bao. 2007 Aug 25;59(4):465-76. Sheng Li Xue Bao. 2007. PMID: 17700966
-
Dynamic regulation of CFTR bicarbonate permeability by [Cl-]i and its role in pancreatic bicarbonate secretion.Gastroenterology. 2010 Aug;139(2):620-31. doi: 10.1053/j.gastro.2010.04.004. Epub 2010 Apr 14. Gastroenterology. 2010. PMID: 20398666
-
SLC26 anion exchangers in uterine epithelial cells and spermatozoa: clues from the past and hints to the future.Cell Biol Int. 2014 Jan;38(1):1-7. doi: 10.1002/cbin.10183. Epub 2013 Oct 17. Cell Biol Int. 2014. PMID: 24115633 Review.
-
Negative News: Cl- and HCO3- in the Vascular Wall.Physiology (Bethesda). 2016 Sep;31(5):370-83. doi: 10.1152/physiol.00001.2016. Physiology (Bethesda). 2016. PMID: 27511463 Review.
Cited by
-
SLC26 Anion Transporters.Handb Exp Pharmacol. 2024;283:319-360. doi: 10.1007/164_2023_698. Handb Exp Pharmacol. 2024. PMID: 37947907 Review.
-
Targeting Solute Carrier Transporters (SLCs) as a Therapeutic Target in Different Cancers.Diseases. 2024 Mar 21;12(3):63. doi: 10.3390/diseases12030063. Diseases. 2024. PMID: 38534987 Free PMC article. Review.
-
CFTR is required for the migration of primordial germ cells during zebrafish early embryogenesis.Reproduction. 2018 Sep;156(3):261-268. doi: 10.1530/REP-17-0681. Epub 2018 Jun 21. Reproduction. 2018. PMID: 29930176 Free PMC article.
-
Estrogen's Tissue-Specific Regulation of the SLC26A6 Anion Transporter Reveal a Phenotype of Kidney Stone Disease in Estrogen-Deficient Females: A Systematic Review.Cureus. 2023 Sep 24;15(9):e45839. doi: 10.7759/cureus.45839. eCollection 2023 Sep. Cureus. 2023. PMID: 37881392 Free PMC article. Review.
-
What Role Does CFTR Play in Development, Differentiation, Regeneration and Cancer?Int J Mol Sci. 2020 Apr 29;21(9):3133. doi: 10.3390/ijms21093133. Int J Mol Sci. 2020. PMID: 32365523 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

