Quantitative analysis of co-oligomer formation by amyloid-beta peptide isoforms
- PMID: 27346247
- PMCID: PMC4921824
- DOI: 10.1038/srep28658
Quantitative analysis of co-oligomer formation by amyloid-beta peptide isoforms
Abstract
Multiple isoforms of aggregation-prone proteins are present under physiological conditions and have the propensity to assemble into co-oligomers with different properties from self-oligomers, but this process has not been quantitatively studied to date. We have investigated the amyloid-β (Aβ) peptide, associated with Alzheimer's disease, and the aggregation of its two major isoforms, Aβ40 and Aβ42, using a statistical mechanical modelling approach in combination with in vitro single-molecule fluorescence measurements. We find that at low concentrations of Aβ, corresponding to its physiological abundance, there is little free energy penalty in forming co-oligomers, suggesting that the formation of both self-oligomers and co-oligomers is possible under these conditions. Our model is used to predict the oligomer concentration and size at physiological concentrations of Aβ and suggests the mechanisms by which the ratio of Aβ42 to Aβ40 can affect cell toxicity. An increased ratio of Aβ42 to Aβ40 raises the fraction of oligomers containing Aβ42, which can increase the hydrophobicity of the oligomers and thus promote deleterious binding to the cell membrane and increase neuronal damage. Our results suggest that co-oligomers are a common form of aggregate when Aβ isoforms are present in solution and may potentially play a significant role in Alzheimer's disease.
Figures

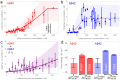

 is unchanged from the single-species value (in which case the ratio of Aβ40:Aβ42 is irrelevant). The true distribution will decline with oligomer size even more rapidly, as visual inspection of the data shows
is unchanged from the single-species value (in which case the ratio of Aβ40:Aβ42 is irrelevant). The true distribution will decline with oligomer size even more rapidly, as visual inspection of the data shows  to be less favourable than the single-species values. The error bars correspond to averaged uncertainty in the ΔG measurements. (c) The relative concentration of oligomers estimated to be bound to the surface of a neuronal cell, expressed relative to the concentration of oligomers bound to the surface at 1 nM of Aβ40. This result assumes that the relative affinity of co-oligomers for the cell membrane is 2 times higher than the affinity of Aβ40 oligomers, and that the relative affinity of Aβ42 oligomers is 4 times higher than that of Aβ40 oligomers.
to be less favourable than the single-species values. The error bars correspond to averaged uncertainty in the ΔG measurements. (c) The relative concentration of oligomers estimated to be bound to the surface of a neuronal cell, expressed relative to the concentration of oligomers bound to the surface at 1 nM of Aβ40. This result assumes that the relative affinity of co-oligomers for the cell membrane is 2 times higher than the affinity of Aβ40 oligomers, and that the relative affinity of Aβ42 oligomers is 4 times higher than that of Aβ40 oligomers.References
-
- Selkoe D. J. Alzheimer’s disease: genes, proteins, and therapy. Physiol Rev 81, 741–766 (2001). - PubMed
-
- Jarrett J. T., Berger E. P. & Lansbury P. T. The carboxy terminus of the beta amyloid protein is critical for the seeding of amyloid formation: implications for the pathogenesis of Alzheimer’s disease. Biochemistry 32, 4693–4697 (1993). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

