Distinct Subunit Domains Govern Synaptic Stability and Specificity of the Kainate Receptor
- PMID: 27346345
- PMCID: PMC4963241
- DOI: 10.1016/j.celrep.2016.05.093
Distinct Subunit Domains Govern Synaptic Stability and Specificity of the Kainate Receptor
Abstract
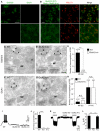
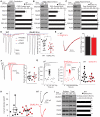
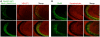
Synaptic communication between neurons requires the precise localization of neurotransmitter receptors to the correct synapse type. Kainate-type glutamate receptors restrict synaptic localization that is determined by the afferent presynaptic connection. The mechanisms that govern this input-specific synaptic localization remain unclear. Here, we examine how subunit composition and specific subunit domains contribute to synaptic localization of kainate receptors. The cytoplasmic domain of the GluK2 low-affinity subunit stabilizes kainate receptors at synapses. In contrast, the extracellular domain of the GluK4/5 high-affinity subunit synergistically controls the synaptic specificity of kainate receptors through interaction with C1q-like proteins. Thus, the input-specific synaptic localization of the native kainate receptor complex involves two mechanisms that underlie specificity and stabilization of the receptor at synapses.
Copyright © 2016. Published by Elsevier Inc.
Figures

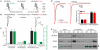

References
-
- Bats C, Groc L, Choquet D. The interaction between Stargazin and PSD-95 regulates AMPA receptor surface trafficking. Neuron. 2007;53:719–734. - PubMed
-
- Castillo PE, Malenka RC, Nicoll RA. Kainate receptors mediate a slow postsynaptic current in hippocampal CA3 neurons. Nature. 1997;388:182–186. - PubMed
-
- Chen L, Chetkovich DM, Petralia RS, Sweeney NT, Kawasaki Y, Wenthold RJ, Bredt DS, Nicoll RA. Stargazin regulates synaptic targeting of AMPA receptors by two distinct mechanisms. Nature. 2000;408:936–943. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

